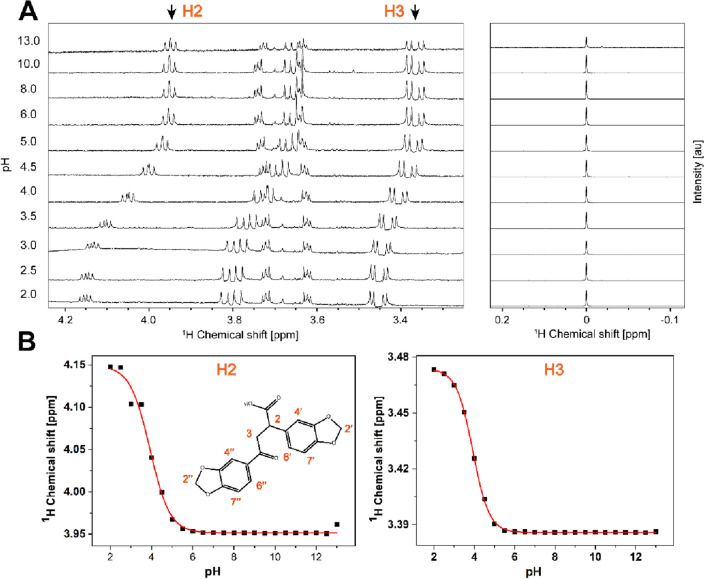
Figure 6.
Determination of the pKa value for 7.44 by 1H NMR. (A) 1H NMR spectra of 200 µM of 7.44 measured from pH 2 to 13 at 298 K at 600 MHz. Sodium 2,2-dimethyl-2-silapentane-5-sulfonate (DSS) was used for chemical shift referencing and calibrated to a zero ppm value. (B) Titration curves obtained from the 1H chemical shifts of the protons H2 and H3 as a function of pH. The pKa values were calculated using the Henderson-Hasselbalch equation25, yielding a pKa value of 3.99 ± 0.01 and 3.93 ± 0.01 of the carboxylic group as indicated by proton H2 and H3, respectively.