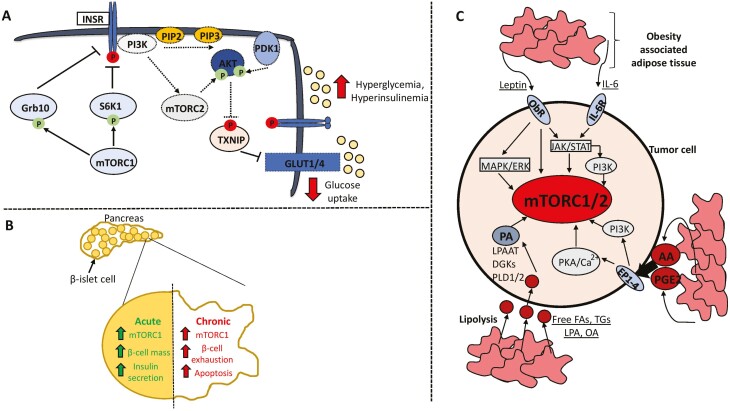
Figure 2.
mTOR signaling in the context of type 2 diabetes and obesity. Hyperactivation of mTOR in response to chronic nutrient excess contributes to type 2 diabetes through 2 main pathways. (A) Sustained mTORC1 activity induces the negative-feedback loops converging on activation of the insulin receptor and downstream induction of the mTORC2–AKT axis that promotes glucose uptake. This contributes to hyperglycemia and hyperinsulinemia. (B) Chronic mTORC1 activation leads to exhaustion and apoptosis of pancreatic β-islet cells, culminating in reduced insulin secretion. (C) Obesity-associated adipose tissue profoundly alters the tumor microenvironment and can stimulate mTOR activity through secretion of adipokines such as leptin and IL-6, proinflammatory metabolites including arachidonic acid and prostaglandin E2, and release of fatty acid substrates through lipolysis that can be used to synthesize phosphatidic acid. Activating and inhibitory phosphorylation events are denoted by an arrowhead or blunt ended lines, respectively. Dotted lines or outlines surrounding enzymes denote downregulation/inhibition. Abbreviations: T2D, type 2 diabetes; INSR, insulin receptor; Grb10, growth factor receptor bound protein 10; PIP2, phosphatidylinositol (4,5)-bisphosphate; PIP3, phosphatidylinositol (3,4,5)-trisphosphate; PDK1, phosphoinositide-dependent kinase 1; TXNIP, thioredoxin interacting protein; ObR, leptin receptor; IL-6, interleukin-6; PKA, protein kinase A; AA, arachidonic acid; PGE2, prostaglandin E2; FA, fatty acid; TG, triglycerides; LPA, lysophosphatidic acid; OA, oleic acid; PA, phosphatidic acid; PLD1/2; phospholipase D1/2; DGKs, diacylglycerol kinase; LPAAT, lysophosphatidic acid acyltransferase.