Abstract
Dopamine (DA, 3-hydroxytyramine) is a member of the catecholamine family and is classically characterized according to its role in the central nervous system as a neurotransmitter. In recent decades, many novel and intriguing discoveries have been made about the peripheral expression of DA receptors (DRs) and the role of DA signaling in both normal and pathological processes. Drawing from decades of evidence suggesting a link between DA and cancer, the DA pathway has recently emerged as a potential target in antitumor therapies. Due to the onerous, expensive and frequently unsuccessful nature of drug development, the repurposing of dopaminergic drugs for cancer therapy has the potential to greatly benefit patients and drug developers alike. However, the lack of clear mechanistic data supporting the direct involvement of DRs and their downstream signaling components in cancer represents an ongoing challenge that has limited the translation of these drugs to the clinic. Despite this, the breadth of evidence linking DA to cancer and non-tumor cells in the tumor microenvironment justifies further inquiry into the potential applications of this treatment modality in cancer. Herein, we review the literature characterizing the interplay between the DA signaling axis and cancer, highlighting key findings, and then propose rational lines of investigation to follow.
Introduction
Dopamine (DA, 3-hydroxytyramine) and its associated receptors comprise a signaling network that has been extensively characterized in many vital central nervous system (CNS) processes over the past 65 years. These functions include, but are not limited to, voluntary movement, reward, sleep regulation, feeding, mental affect, attention, cognitive function, olfaction, vision, hormonal regulation and sympathetic regulation (1). DA is a catecholamine neurotransmitter and a precursor to norepinephrine and epinephrine.
Like other neurotransmitters, DA signaling is mediated through a complex network of G-protein-coupled receptors (GPCRs), downstream effector molecules and metabolizing enzymes that are present in both the CNS and the periphery. These DA receptors (DRs) are divided into two groups: D1-like and D2-like receptors (D1Rs and D2Rs, respectively) (2). The D1Rs—DRD1 and DRD5—generally associate with the Gαs/olf subunit and activate adenylyl cyclase. Conversely, D2Rs—DRD2, DRD3 and DRD4—typically partner with the Gαi/o subunit and inhibit adenylyl cyclase activity (3,4).
Early evidence that DA signals outside of the CNS came from the discovery of its vasodilatory effect in renal tissue (5). Since then, DA has been implicated in various tissues including vascular beds, heart, gastrointestinal (GI) tract, eye, kidney and pancreas (6–9). As will be discussed in greater detail later in this review, DRs are also expressed in a variety of immune cell subsets, where they regulate differentiation and activation, functions that could be relevant to anticancer immunotherapeutic strategies (10,11).
There is now an increasing body of evidence that DA signaling in peripheral tissues is perturbed in cancer and this nascent field offers novel insights into cancer cell vulnerabilities and underscores the potential to both purpose and repurpose the libraries of dopaminergic ligands and drugs that arose from neuropharmacological drug discovery and development (12). In this review, we will highlight epidemiological, molecular and clinical evidence of the DA pathway’s involvement in cancer and provide our perspective on how the role of DA in tumors and in the tumor microenvironment (TME), particularly within the immune system, can be translated for the therapeutic benefit of cancer patients.
DA-related pathologies and therapeutics linked to cancer
Parkinson’s disease and cancer
In 1957, the pioneering neuropharmacologist Avid Carlsson published the first observation that DA functions as a classical neurotransmitter within the CNS, independent of its role as a precursor to epinephrine and norepinephrine—a discovery for which he would ultimately share the 2000 Nobel Prize in Physiology or Medicine (13,14). Shortly after Carlsson’s seminal paper on DA, Ehringer and Hornykiewicz found that the antihypertension and antipsychotic drug, reserpine, depletes DA in the brain and induces a Parkinson’s disease (PD) phenotype (15). Intravenous administration of the DA precursor, 3,4-dihydroxyphenylalanine (l-DOPA), followed in 1961 and significantly reduced symptoms in PD patients (16), where orally administered l-DOPA remains the standard course of treatment (17). l-DOPA is used over DA as the latter cannot cross the blood–brain barrier.
In the years since, physicians and epidemiologists have reported several interesting connections between PD and cancer. PD is a neurodegenerative disorder characterized by loss of dopaminergic neurons in the substantia nigra pars compacta, depletion of DA in the striatum and the presence of Lewy bodies. Cancer is a neoplastic disorder characterized by uncontrolled cell growth and lack of cell death in the affected tissue (18,19). Thus, at a cellular level, PD and cancer are diseases of fundamentally opposite manifestation. At the population level, many epidemiological analyses have also revealed an inverse relationship between the incidence of PD and cancer mortality (20–24), but the results have not always been consistent and may vary with tumor type (25,26).
A recent meta-analysis characterized the risk of lung cancer in 15 studies comprising 348 780 PD patients and found that PD patients had a 47% reduction in risk of developing lung cancer (22). Importantly, the timing of diagnosis was critical for observing this effect, as only the patients diagnosed with PD had a reduced risk of subsequent lung cancer diagnosis, which, the authors suggested, could be explained by exposure to dopaminergic therapy. Another large study examining the relationship between PD and cancer using data from Surveillance, Epidemiology, and End Results (SEER) and Medicare found that the odds of lung cancer in the total population after a PD diagnosis was reduced, consistent with the previous paper. However, the authors also noted that the reduced odds of a cancer diagnosis after a PD diagnosis was similar to the reduced odds of a cancer diagnosis after an automobile crash. As cancer and automobile crashes are unlikely to be biologically related, the authors concluded that the association between PD and subsequent cancer risk was consistent with a bias in ascertaining cancer in patients with other serious medical conditions rather than an association between PD and cancer (26).
Melanoma, however, has the opposite trend and has been consistently demonstrated to co-occur with PD at significantly higher than expected rates (27–31). The largest prospective study of melanoma in PD to date contained 2106 PD patients and found that PD patients’ relative risk for melanoma was seven times greater than predicted based on the American Academy of Dermatology skin cancer screening programs (30). Unlike with lung cancer, the association between PD and melanoma is independent of the timing of diagnosis—melanoma patients are at 50% greater risk of subsequent PD diagnosis (28), and PD patients are two times more likely to be subsequently diagnosed with melanoma (27). Thus, it appears unlikely that l-DOPA therapy for PD mediates the increased risk for melanoma (32,33). Additionally, individuals who did not have melanoma but did have a family history of melanoma were twice as likely to be diagnosed with PD than those without family history of melanoma (29). However, melanoma patients without PD are 10.5 times more likely to die of metastatic melanoma than melanoma patients who have PD, suggesting PD-linked melanoma may be less aggressive (34). This unusual relationship between melanoma and PD likely relates to their shared melanin dysfunction. Melanogenesis is an enzymatic process dependent on l-DOPA that is commonly dysregulated in melanocytes of a melanoma tumor. Conversely, PD is characterized by the degeneration of melanin-rich DA-producing neurons. The complex relationship between melanoma and PD and the potential role of DA signaling in reducing melanoma progression deserves further inquiry.
DRD2-related psychotropics and schizophrenia and cancer
Although DA research progressed slowly for the decade following its discovery in PD in 1957, a concurrent breakthrough in psychiatry during the 1950s lead to some of the earliest documented connections between DA and cancer (35). In 1952, the antipsychotic effects of the DRD2 antagonist, chlorpromazine (aka Thorazine), were discovered, allowing the drug to be prescribed for millions of psychiatric patients (36,37). The subsequent propagation of DA blockers in the clinic drove several interesting revelations about the pathological relevance of the DA pathway. Among them was the DA hypothesis of schizophrenia, which postulates that excessive DA signaling mediated by excessive D2R and reduced D1R activity drives the positive and negative disease symptoms, respectively (38–40). Treatment of schizophrenic patients with DRD2 antagonists has led to multiple interesting but complex links between DA signaling and cancer.
Isolated case reports from the 1960s and 1970s suggested that there was increased response to radiation and chemotherapy in cancer patients concurrently treated with antipsychotics (41–43), prompting the hypothesis that the biological processes by which these drugs work could somehow impact cancer risk, development and/or progression. As with the studies examining PD and cancer, the literature is heterogeneous, and few unequivocal or clear trends emerge (44–47). Some factors that could impact the lack of a unifying hypothesis in these studies is the use of variable methodologies (prospective versus case–control) and sample sizes (48,49), background co-morbidity of the participants, heterogeneity in molecular subtypes and therapy received.
Two relatively common SNPs in the DRD2 gene, rs1799732 and rs1800497, which are associated with PD and schizophrenia, are significantly associated with increased colorectal cancer (CRC) risk (50) and adenoma recurrence (51). These polymorphisms are thought to reduce both the expression and function of DRD2 (52–54). Although reduced DRD2 expression has been observed in cancers of the stomach and colon (55,56), the impact of these SNPs on DRD2 expression in epithelial cells of the GI tract has not been analyzed. Inherited mutations in DA-related genes have also been linked with cancer. Our group recently demonstrated that a SNP in DRD1 was associated with lung cancer risk (57). We initially thought this association was related to nicotine addiction given that nicotine mediates its reward effects via DA. Nicotine binds to nicotinic receptors on dopaminergic neurons and causes the release of DA. However, as we also found this relationship among never smokers, our results suggested a direct link between DA with lung cancer biology. Similarly, the studies on D2R SNPs mentioned above also did not find evidence of an interaction between the reward pathway and these SNPs, again suggesting a direct biological link between DRD2 function and cancer risk (50,51,58).
As described above, the relationship between psychiatric disorders and antipsychotic medications with cancer is still unclear based on the current literature (59,60). In addition to timing, the dosage and reasons for drug administration could affect the relationship with cancer. Further, the pleiotropic manner in which these drugs work, coupled with the expression of altered forms of the DRs, could also confound epidemiological studies. For example, alternative splicing of DR genes has been reported, including DRD2, a D2R family member that is frequently the target of antipsychotic drugs. DRD2 exists as two main isoforms, denoted as long (D2L) and short (D2S), that differ by 29 amino acids (61). Interestingly, these two isoforms impart widely varying phenotypic effects and reflect different binding affinities with DA agonists (62,63). Further, several functional germline genetic variants in DA-related genes have been linked with schizophrenia, hypertension, cancer risk and renal tubule dysfunction (64–67), which could also impact and/or confound population-based studies. As will be discussed in greater detail in section S2.0 of the Supplementary Material, DA—and indeed germline variants in DR genes—impact the immune system, highlighting another layer of complexity unlikely to be accounted for in etiological epidemiological studies.
Although epidemiologic studies suggest a complex and at times conflicting relationship between DA signaling and cancer, there is growing molecular and clinical evidence for a direct, biological relationship between the peripheral DA signaling network and cancer in terms of risk, development and progression. The purpose of this review is to present this literature and examine the basis for leveraging this developing field for new treatment directions.
Molecular evidence for mechanisms of DA activity in cancer and translation to clinical studies
The first demonstration of DA’s impact on cancer cells was over four decades ago when DA inhibited the growth of B16 melanoma cells (68). While, overall, the mechanisms by which DA elicits tumor suppressive effects remain poorly defined, recent target-agnostic high-throughput drug screens have frequently identified DA-related modulators as anticancer targets. This section discusses the potential therapeutic impact of various DR modulators in cancer as evidenced in a variety of key studies in both the preclinical and clinical settings. For a detailed discussion of crosstalk and therapeutic synergies between DA signaling and other relevant pathways, including somatostatin and insulin-like growth factor-1 (IGF-1), please refer to section S1.0 of the Supplementary Material.
Preclinical studies, phase 1 and 2 trials: D2-like targeting
Preclinical studies of D2R targeting agents
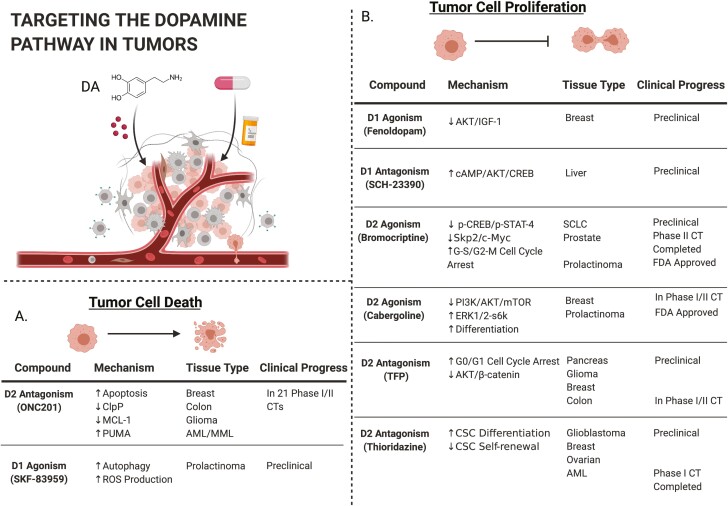
Early observations describing the link between antipsychotic drugs and response to cancer therapy (41–43,69) gained important mechanistic validation in 2014 when a phenotypic screen of 1974 small molecules identified trifluoperazine (TFP) as an antimetastatic agent (70). TFP is a well-characterized DRD2 antagonist that is generally well tolerated by schizophrenic patients (71). Since then, TFP has demonstrated preclinical efficacy in a variety of tumor types, including triple-negative breast cancer, pancreatic ductal adenocarcinoma (PDAC), glioblastoma (GBM), CRC and lung cancer (72–76). TFP inhibits migration via a DRD2/AKT/β-catenin signaling axis. It reduces activating phosphorylation marks on AKT and β-catenin, preventing β-catenin nuclear translocation and transcriptional activation of angiogenic and antiapoptotic genes (Figure 1) (72,76).
Figure 1.
Targeting the dopamine pathway in tumor cells. Upper left: schematic of a solid tumor with tumor cells and associated non-tumor cells. (A) Description of compounds in development that promote cell death as their primary mechanism of action. (B) Description of compounds in development that inhibit cell proliferation as their primary mechanism of action. Image created with Biorender.com.
Inhibition of DRD2 with TFP also inhibits the growth of CRC cell lines in vitro and in vivo by inducing cell cycle arrest at G0/G1 (76). In addition, TFP promotes mitochondria-mediated intrinsic apoptosis and targets the DR pathway-related molecules, calmodulin and Forkhead Box Protein O1 (FOXO1) (77). Most recently, TFP was shown to enhance the efficacy of radiation therapy in a murine GBM model by inhibiting the phenotypic conversion of glioma-initiating cells to transformed cells (78).
DRD2 antagonism via other pharmacological compounds, including Pimozide and ONC201, have also shown anticancer activity in a variety of other contexts, including PDAC and GBM, through a mechanism that involves activation of the cAMP/PKA pathway, at least partially (79–82). It is worth noting, however, that activation of DRD2, as opposed to antagonism, also inhibits cell proliferation in several tumor types, including breast and lung cancer (83). As is currently the case with other DR modulators that have demonstrated activity in the cancer setting, the precise signaling mechanisms by which these DRD2 modulating drugs exert their anticancer effects are not clearly delineated at present.
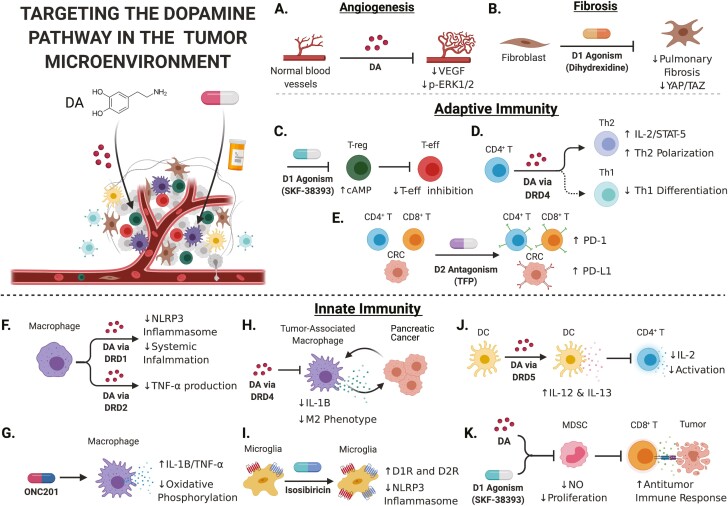
DA is a potent inhibitor of angiogenesis through DRD2 signaling (84,85). Angiogenesis, or the formation of new blood vessels, is essential in supporting tumor growth and progression (86,87). DA administration inhibits tumor growth and vascularization in multiple mouse tumor models through DRD2 agonism leading to inhibition of VEGFA-mediated ERK1/2 phosphorylation in endothelial cells (Figure 2A) (88). It also has reduced renal and cardiovascular toxicities compared to treatment with another antiangiogenic drug, sunitinib (89).
Figure 2.
Targeting the dopamine pathway in the tumor microenvironment. Note: For a detailed discussion of the interactions between DA signaling and the immune system, including the mechanisms depicted in this figure, please refer to section S2.0 of the Supplementary Material. Upper left: Schematic of a solid tumor with tumor cells and associated non-tumor cells. (A) DA treatment inhibits angiogenesis by reducing VEGF signaling and ERK phosphorylation in endothelial cells. (B) DRD1 agonism with dihydrexidine regulates YAP/TAZ signaling in lung fibroblasts to reverse idiopathic pulmonary fibrosis. (C) Treating regulatory T cells with DRD1 agonist, SKF-38393, upregulates cAMP production and reduces their immunosuppressive regulation of effector T cells. (D) Activation of DRD4 on CD4 T cells via DA treatment increases production of IL-2 and STAT-5, and promotes their differentiation to T-helper 2 phenotype. (E) Treatment of colorectal cancer cells with DRD2 antagonist, TFP, increased PD-L1 expression in vitro and in vivo. TFP treatment also increased PD-1 expression on tumor-infiltrating T cells. (F) DA treatment inhibits NLRP3 inflammasome activation in macrophages and reduces systemic inflammation in mice, both in a DRD1-dependent manner. DA treatment also reduces production of the proinflammatory cytokine, TNF-α, in macrophages. (G) ONC201 treatment represses oxidative phosphorylation in macrophages and promotes secretion of IL-1B and TNF-α. (H) Activation of DRD4 on tumor-associated macrophages (TAMs) via DA treatment reduces the secretion of protumor cytokines and reduces the tumor-protective effects associated with the M2 phenotype. (I) Treating microglia with isosibiricin increased expression of DRD1 and DRD2 and reduced activation of the NLRP3 inflammasome. (J) Activation of DRD5 on dendritic cells via DA treatment increased secretion of IL-12 and IL-13, and also inhibited CD4 T-cell activation and IL-2 production. (K) Treatment with DA and SKF-38393 inhibited myeloid-derived suppressor cell activity, as measured by nitric oxide production, proliferation and inhibition of CD8 T-cell interaction with tumor cells. Image created with Biorender.com.
Interestingly, another anticancer phenotypic neurochemical screen in GBM revealed that DRD4 antagonism, by PNU 96415E, reduced GBM stem cell proliferation and survival through inhibition of the autophagy-lysosomal degradation pathway while also promoting neuronal stem cell differentiation into normal cells (90). The authors also reported that PNU 96415E did not affect the viability of fibroblasts, which were used in this screen as a control for non-specific cytotoxic effects. It has since been shown that fibroblasts can be regulated by the DA pathway through DRD1 (91), spotlighting the DRD4-specific activity of PNU 96415E in this screen. In addition to GBM stem cells, D2Rs are also a strong drug target in other cancer stem cells. While normal human pluripotent stem cells (hPSCs) are devoid of DR expression, all five DRs are present in neoplastic hPSCs, making them susceptible to DRD2 modulation in leukemia and lung cancer (83,92).
In addition to DRD2 and DRD4, DRD3 has also shown potential as a drug target in preclinical studies. Cariprazine, a DRD3 partial agonist used to treat schizophrenia and bipolar disorder, inhibits multidrug resistance and sensitizes colon and lung cancer cells to antineoplastic drugs (93). Cariprazine is an atypical antipsychotic drug because of its unique pharmacodynamic activity—it partially agonizes DRD2, DRD3 and 5-HT1A receptors and antagonizes 5-HT2B receptors (94). It also shows moderate affinity for adrenergic, histaminergic and cholinergic receptors, which allows this drug to alleviate many of the side effects associated with conventional antipsychotic drugs, which will be important to consider when repurposing these drugs for cancer treatment.
Thus far, we have described the anticancer activity of several D2R targeting compounds in preclinical models. We feel that these compounds provide important proof-of-concept and mechanistic data, which together support the implementation of dopaminergic drugs in cancer treatment modalities. While some of these compounds continue to be characterized and advanced to clinical trials, we will review the efficacy of several other dopaminergic drugs that have already progressed through clinical development in the next section.
Phase 1 and 2 clinical trials
Cabergoline
Cabergoline, a semisynthetic ergot alkaloid DRD2 agonist, is the current treatment of choice for patients with prolactinoma (95). Prolactinoma is a non-cancerous adenoma of the pituitary gland and represents the most common type of hormonally active pituitary tumor. Pituitary tumors, which typically have high D2R expression, have been successfully treated by ergot alkaloid DRD2 agonists for nearly 50 years. Not only does DRD2 agonism reduce tumor size in prolactinoma, it reduces secretion of the lactotropic hormone, prolactin (PRL), and mitigates subsequent gonadal dysfunction (96).
DA/DRD2 signaling negatively regulates the transcription of PRL through its inhibition of the cAMP/PKA pathway (97–99). Persistent ERK activation by DRD2 in lactotropes promotes cell differentiation rather than proliferation, which ultimately hinders tumor growth. Agonizing DRD2 with cabergoline functionally offsets the balance between the ERK1/2 and PI3K pathways in prolactinoma cells, inhibiting PI3K/AKT/MTOR-mediated proliferation and promoting ERK/S6K-mediated differentiation (63).
Given the role of DRD2 and DA agonists in regulating PRL secretion and milk production, there is also interest in understanding the significance of this pathway in breast cancer. As many antipsychotics increase serum levels of PRL through antagonism of inhibitory D2Rs of lactotropes, this could account for the increased risk of breast cancer among female schizophrenic patients described above (48,49). Indeed, cabergoline is being evaluated in both phase I and II clinical trials for treatment of metastatic breast cancer (mBC) (100–102). Early results from a phase II study of 20 hormone receptor-positive, mBC patients indicated that cabergoline was well tolerated and that it provided clinical benefit to 33% of evaluable patients (101). While patient response to cabergoline was lower than expected in this trial, its safety profile and partial benefit provided to patients support its use in certain patient subsets stratified by DR expression and in combination with other therapies.
Bromocriptine
As a DRD2 agonist, bromocriptine is approved for the treatment of PD, neuroleptic malignant syndrome, and type 2 diabetes mellitus (103–105). Prior to cabergoline’s success in treating prolactinoma, bromocriptine was the preferred course of treatment for prolactinoma for over two decades. Although cabergoline is considerably more effective in reducing symptoms of prolactinoma such as amenorrhea, galactorrhea and lower pregnancy, there is very little evidence that it is better at controlling prolactinoma growth (106). Bromocriptine has also shown promise in preclinical models of various other cancer types. Bromocriptine inhibits the growth of xenografts from small cell lung cancer patients in a DRD2-dependent manner (107). In an independent screen of FDA-approved small molecules, bromocriptine was recently identified as a candidate to treat acute myeloid leukemia (AML) (108). Validation studies revealed that it selectively activated apoptosis and induced myeloid differentiation of the primitive CD34+/CD38− leukemic stem cell fraction in AML patient primary samples (108,109).
Most recently, bromocriptine enhanced the efficacy of docetaxel, the standard-of-care chemotherapy for treating prostate cancer (PCa) bone metastases in the C4-2 mouse model by inducing cell cycle arrest at both the G1-S and G2-M phases (110). DRD2 is frequently expressed in human PCa and is reduced in patients with higher Gleason scores. Consistent with previous work, bromocriptine reduced phosphorylation of the downstream transcriptional activators, CREB and STAT4, but did not affect phosphorylation of AKT, which is commonly modulated by other DRD2 drugs. Other molecular targets of bromocriptine, which include androgen receptor, Skp2, p53, c-Myc and survivin, are potential surrogate biomarkers to evaluate the clinical effectiveness of bromocriptine in humans. The findings of this study are interesting in the context of several other phase 1 and 2 trials, launched decades earlier, that investigated the efficacy of bromocriptine in treating castration-resistant PCa. The overall results were that the drug was safe and well tolerated; 50% of patients’ bone pain was alleviated immediately, and response rates were variable ranging from 0 to 22% regression, but notably these patients were not stratified by DR expression (111–113). These studies are a reminder that the tissue-specific effects of DRs and their pharmacological ligands must be clarified in detail to more accurately assess the efficacy and molecular mechanism of action within each cancer type.
Thioridazine
The antipsychotic agent thioridazine, which was first observed to sensitize cancer patients to radiation in 1988, antagonizes all DRs although it has highest affinity for DRD2. Its anticancer activity has since been independently validated in numerous studies, including three agnostic screening approaches (92,114,115). It induces differentiation of cancer stem cells in AML (92) and enhances the efficacy of the relatively toxic standard-of-care chemotherapeutic, cytarabine. This observation led to a phase 1 clinical trial evaluating the safety and feasibility of oral thioridazine combined with cytarabine in difficult-to-treat, elderly AML patients with relapsed or refractory disease (116). There, thioridazine had efficacy as a monotherapy and reduced leukemic burden in 8 out of 11 patients, with reductions ranging from 19 to 55%. The effect of thioridazine was correlated with patient-specific levels of cellular DRD2 expression, suggesting that DRD2 expression could be a companion biomarker. Furthermore, these responses were observed after only a 5-day exposure to thioridazine as a monotherapy; the leukemic blast count began to increase again once dosing stopped. Although dose-limiting neurolepsis and cardiotoxicity hinder longer dosing schedules (117), possible solutions include drug reformulation, gradual dose escalation and selection of younger trial participants. While this approach needs to be evaluated in larger and more diverse clinical trials, ideally using a more bioavailable formulation of the drug, this study provides an important preliminary signal that DR antagonism is a viable clinical approach to cancer therapy.
It should be noted, however, that the role of DRD2 in the activity of thioridazine has not yet been validated using stringent knockout models, and thus, should be further elucidated in order to maximize the drug’s potential clinical utilization. Although thioridazine has high affinity for DRD2, it also antagonizes other catecholamine receptors, including adrenergic receptors, which could contribute to the observed antileukemic effects. Nonetheless, this compound has been identified in several anticancer drug screens, and its mechanism of action reflects many similarities shared by most DR modulators in cancer. Using the Connectivity Map, a collection of genome-wide transcriptional datasets from cultured human cancer cell lines, Rho et al. predicted that thioridazine treatment produces a gene expression signature similar to those of AKT inhibitors (114). Follow-up in vitro experiments verified that thioridazine treatment reduced phosphorylation of AKT, induced apoptosis and enhanced cytotoxicity when combined with standard-of-care cisplatin treatment. An independent study using a genome-wide RNA interference screen for therapeutic partners of the EGFR inhibitor, gefitinib, in lung cancer produced very similar results implicating thioridazine as a synthetic lethal partner (115).
ONC201
ONC201 is a first-in-class member of the imipridone molecular family that acts as a DRD2 antagonist and is currently being evaluated for clinical use in various types of cancers (12,80,118–121). Collectively, the preclinical efficacy of ONC201 in several tumor types was promising enough to guide the establishment of several dozen phase I and II clinical trials, some of which will be discussed further below. Along with its noteworthy progress in the clinic— there are at least 24 clinical trials currently underway—the detailed molecular studies of this novel compound’s anticancer activity have revealed that although DRD2 antagonism is a component, its mechanism is complex, tissue type-dependent and likely involves multiple targets.
ONC201 was first described as a TNF-related apoptosis-inducing ligand (TRAIL) activator, as it was found to promote cell death through TRAIL/death receptor (DR)-5 upregulation and enhance chemotherapeutic efficacy in CRC cells in a TRAIL-based drug screen (12,118,122). Subsequent characterization of ONC201 revealed that DRD2 and DRD3 are its direct molecular targets, with TRAIL/DR-5 upregulation being a downstream consequence. However, DRD3 is rarely expressed in human tumors, while DRD2 is frequently overexpressed (123). Consistent with many of the other DRD2 modulators described in this review, early data showed that ONC201 inhibits ERK and AKT downstream of target engagement, which promotes a proapoptotic phenotype due to increased transcriptional activation of TRAIL/DR-5 by Foxo3 (124). In addition to molecular data, a novel machine-learning analysis of drug screen data also predicted that ONC201 would antagonize DRD2 (125). In line with these studies, CRISPR-mediated knockout of DRD2 also promoted ERK inhibition in GBM cells. These data support ONC201’s modulation of the canonical DRD2/cAMP/p38 signaling pathway. However, notably unlike other antipsychotic DRD2 antagonists, ONC201 has a lower affinity and higher fidelity for DRD2 and, importantly, a wider therapeutic index (126).
Aside from its unique kinetic properties, there are several key differentiating factors that separate ONC201 from other DRD2 modulators, and also suggest the engagement of other targets. In several cancer types, ONC201 consistently upregulates the integrated stress response (ISR) by activating ATF4/CHOP in several cancer types; however, ISR activation is not a common feature of DRD2 perturbation (127,128). Nonetheless, the synergistic activation of the ISR through ATF4 and the inactivation of the AKT/ERK signaling cascade can both promote apoptosis through TRAIL/DR-5 upregulation, with the former mechanism being an early effect observed within 2–3 h of treatment and the latter being a latent effect observed within 48–72 h (124,128).
Furthermore, when DRD2 expression was knocked out in three breast cancer cell lines, the anticancer effects of ONC201 were not abrogated. However, it was shown that overexpression of DRD2 did enhance the proapoptotic effects of ONC201, as measured by CHOP induction and subsequent PARP cleavage, suggesting a partial role for DRD2 in ONC201’s effects (80). A phase II clinical trial of ONC201 in 17 patients with recurrent GBM, which did not reach its primary endpoint of 6-month progression-free survival, found that patients demonstrated a relatively marginal increase in circulating PRL, which is a classical marker of DRD2 antagonism (129,130), despite all evaluable tumors expressing high levels of DRD2 (131). Lastly, authors involved in this drug’s development proposed that tumor expression of DRD5 was a negative predictor of ONC201 efficacy (126). They leveraged expression data from several sources to show that DRD5, which generally functions opposite to DRD2 given that its activation elicits antiproliferative and proautophagic cell death phenotypes in various cancer cell lines (131), decreased patient response to ONC201 and demonstrated that it functionally reduced ONC201-mediated apoptosis, as measured by PARP cleavage. Although it is not clear how DRD5 function interferes with DRD2 antagonism by ONC201, Prabhu et al. posited that DRD2/DRD5 dimerization may be involved (126). Additionally, these associations have yet to be directly validated in detail or in a sufficiently large prospective patient cohort. Despite its clinical progress in a multitude of cancer settings, there are important mechanistic underpinnings of ONC201 that remain to be elucidated.
Preclinical studies of D1R targeting agents
D1R targeting
DA signaling through D1Rs affects tumor progression in a variety of cancer types, including an inhibitory effect on cell proliferation and disease progression in colon, breast, prostate and GI cancers (132,133). In breast cancer, where DRD1 expression has previously been linked to advanced disease and poor prognosis (134), DRD1 agonist Fenoldopam inhibited cell proliferation, reduced AKT/IGF-1 activation in tumor cells and abrogated IGF-1 induced endothelial smooth muscle cell growth (critical for angiogenesis) in vitro (133).
DRD1 expression is also significantly upregulated in liver tumors compared to matched non-tumor tissue, and higher expression of DRD1 is associated with significantly lower survival (135). However, DRD1 appears to function as an oncogene in liver cancer, in contrast with the above literature where DRD1 acts like a tumor suppressor. Repression of the Drd1 gene abrogated DA-mediated stimulation of the cAMP/PI3K/AKT/CREB pathway and attenuated cell proliferation and migration in a panel of liver cancer cell lines (135). Further, pharmacological antagonism of DRD1 by SCH-23390 significantly reduced tumor growth in mice. The authors also showed that altered expression of the DA metabolizing enzymes, DOPA decarboxylase and monoamine oxidase A, leads to increased DA production in human liver tumors.
Elsewhere, D1R signaling has been extensively reported in pituitary tumors and prolactinomas. Of note, DRD5 agonism suppresses cell growth through inhibition of mTOR (a downstream target of AKT) and induction of autophagic cell death in pituitary adenoma (136).
D1R targeting may also inhibit tumor growth through modulating fibrosis. Increasing evidence shows that fibroblasts play a critical role in carcinogenesis and disease progression (137). Pharmacological inhibition of DRD1 effectively regulates the vital YAP/TAZ transcriptional co-activator complex in lung fibroblasts to reverse the fatal process of idiopathic pulmonary fibrosis (Figure 2B) (91), which is a risk factor for lung cancer development (138). Fibrosis has also been implicated in a variety of other cancer types including metastatic carcinoma of the skin and PDAC (139,140). Taken together, these data suggest that targeting DRD1 may mitigate the effects of YAP/TAZ activation in tumor types that express D1Rs and selectively inhibit the tumorigenic effects of fibrosis. This mechanism warrants further investigation.
Limitations and future directions of the field
The biological interplay between cancer and neurotransmitters, such as DA, has many relevant clinical and basic science implications. Convincing evidence linking these two ostensibly distinct fields of biology has mounted over the course of the last 70 years, predating the characterization of DA itself. Despite the expansive body of literature establishing connections between various components of the DA signaling network, the dearth of sufficiently detailed mechanistic data elucidating the link between these molecular components and the anticancer activities that they mediate is a limitation of the field (48). At the root of this challenge is the pleiotropic nature of DA signaling and the inherent technical challenges in characterizing DR expression.
As DR receptors can function in opposing ways, it is necessary to understand the expression of all components of the DA network, no matter the tissue or cell type under investigation. This can be limited by the availability of well-characterized antibodies. In cancer, the TME is a diverse and dynamic cellular landscape whose composition ultimately dictates the progression and therapeutic susceptibility of each tumor, and it will be necessary to have a complete view of DA signaling in cancer in various cell types within the TME to implement effective therapies using dopaminergic compounds. Going forward, the use of single cell sequencing platforms could provide necessary insight into the dynamics of DA signaling in the TME to more accurately model the effects of dopaminergic drugs on all cells.
As highlighted in this review, the involvement and activity of DA pathway components in cancer is dynamic and variable. Despite this variation, there are several key consistent trends that lend themselves to the successful adaptation of dopaminergic drugs in treating cancer. First, perturbation of DA signaling is common in many cancer types, affecting tumor growth and patient survival, suggesting that it is a significant manifestation of cancer cell biology. Secondly, DRs have been repeatedly, and independently, implicated as potential antitumor therapeutic targets in at least nine target-agnostic screens (70,81,90,92,108,114,115,125,141). Third, the pharmacological modulation of DA signaling consistently affects cell signaling, survival, proliferation and invasion in cancer cells in vitro and in vivo. It should be noted that there are pharmacodynamic issues in achieving serum concentrations matching those used in vitro to demonstrate efficacy (112), and optimal pharmacodynamic and pharmacokinetic metrics may not be achieved. Many of the anticancer effects caused by DR modulators in vitro can only be observed at high concentrations, greater than 5 μM, which are also likely to promote off-target receptor engagement (136,142). Despite these concerns, the mechanism by which these anticancer effects occur, although not always fully characterized, often involves the same molecular pathways: cAMP/PI3K/AKT and MAPK/ERK. Although some PI3K/AKT and MAPK/ERK inhibitors have gained regulatory approval for cancer, the majority have ultimately failed due to concerns about safety and/or efficacy (143,144). Nonetheless, some of the most impactful targeted therapies recently approved for difficult-to-treat cancers (e.g., melanoma, mBC and non-small cell lung cancer) successfully inhibit these same pathways through interacting with upstream components such as BRAF, HER-2 and EGFR. Thus, DRs represent an attractive approach to potentially enhance efficacy and reduce toxicities associated with AKT- and ERK pathway-directed therapies.
The use of existing datasets to better characterize the expression of the DA network in immune cells will also promote stronger experiments to understand the mechanism by which DA signaling acts on immune cells in the healthy and disease context. The primary translational focus of these studies should be to identify individuals at greater risk of developing cancer and cancer patients who are more likely to respond to immunotherapy, as well as identify potential therapeutic strategies to enhance immunotherapy efficacy through targeting the DA network. More specifically, studies should clarify the physiological source(s) of DA signaling in T cells and identify at what stage(s) their activity is impacted by DA because T cells are either affected by DA signaling during hematopoiesis in lymphoid organs, where adrenergic nerves are present and known to release catecholamines (which has been described in great detail (89,145–148)), or over their lifespan in circulation in peripheral tissues. At present, far less is known about the latter aspect of dopaminergic immunomodulation and what the collective effect of giving a dopaminergic agent to a patient will be on the TME.
It is possible that the DA network will be too complex to target in a cancer cell-specific way. Nonetheless, deeper interrogation of the relationship between DA signaling and cancer could reveal other druggable targets downstream of DRs, or in related pathways, as well as identify subsets of patients that are more responsive to dopaminergic therapies. Despite its complexity and pleiotropy, targeting the DA pathway has demonstrated efficacy across many cancer types, and the arsenal of dopaminergic compounds already in use and under investigation make it a promising avenue for implementing novel cancer therapies.
Supplementary Material
Acknowledgements
C.E.G. is a Predoctoral Fellow in the NIH Graduate Partnership Program supported by the National Cancer Institute in partnership with the Tumor Biology Program of Georgetown University. Dr A.L.F. is a Molecular Pathology Fellow in the NIH Comparative Biomedical Scientist Training Program supported by the National Cancer Institute in partnership with the Comparative Biomedical Sciences Graduate Program of North Carolina State University.
Glossary
Abbreviations:
- AML
acute myeloid leukemia
- CNS
central nervous system
- CRC
colorectal cancer
- DA
dopamine
- DOPA
3,4-dihydroxyphenylalanine
- DR
dopamine receptor
- GI tract
gastrointestinal tract
- GBM
glioblastoma
- hPSC
human pluripotent stem cell
- IGF
insulin-like growth factor
- ISR
integrated stress response
- mBC
metastatic breast cancer
- PCa
prostate cancer
- PD
Parkinson’s disease
- PDAC
pancreatic ductal adenocarcinoma
- PRL
prolactin
- TFP
trifluoperazine
- TME
tumor microenvironment
- TRAIL
TNF-related apoptosis-inducing ligand
Contributor Information
Christopher E Grant, Laboratory of Human Carcinogenesis, Center for Cancer Research, National Cancer Institute, Bethesda, MD, USA.
Amy L Flis, Laboratory of Human Carcinogenesis, Center for Cancer Research, National Cancer Institute, Bethesda, MD, USA.
Bríd M Ryan, Laboratory of Human Carcinogenesis, Center for Cancer Research, National Cancer Institute, Bethesda, MD, USA.
Conflict of Interest Statement
None declared.
References
- 1. Beaulieu, J.M., et al. (2015) Dopamine receptors—IUPHAR review 13. Br. J. Pharmacol., 172, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garau, L., et al. (1978) Dopamine receptors: pharmacological and anatomical evidences indicate that two distinct dopamine receptor populations are present in rat striatum. Life Sci., 23, 1745–1750. [DOI] [PubMed] [Google Scholar]
- 3. Kebabian, J.W. (1978) Multiple classes of dopamine receptors in mammalian central nervous system: the involvement of dopamine-sensitive adenylyl cyclase. Life Sci., 23, 479–483. [DOI] [PubMed] [Google Scholar]
- 4. Spano, P.F., et al. (1978) Studies on the pharmacological properties of dopamine receptors in various areas of the central nervous system. Adv. Biochem. Psychopharmacol., 19, 155–165. [PubMed] [Google Scholar]
- 5. McDonald, R.H.Jr., et al. (1964) Effect of dopamine in man: augmentation of sodium excretion, glomerular filtration rate, and renal plasma flow. J. Clin. Invest., 43, 1116–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beaulieu, J.M., et al. (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev., 63, 182–217. [DOI] [PubMed] [Google Scholar]
- 7. Bucolo, C., et al. (2012) Dopamine-(3) receptor modulates intraocular pressure: implications for glaucoma. Biochem. Pharmacol., 83, 680–686. [DOI] [PubMed] [Google Scholar]
- 8. De Mei, C., et al. (2009) Getting specialized: presynaptic and postsynaptic dopamine D2 receptors. Curr. Opin Pharmacol., 9, 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Missale, C., et al. (1998) Dopamine receptors: from structure to function. Physiol. Rev., 78, 189–225. [DOI] [PubMed] [Google Scholar]
- 10. Basu, S., et al. (1995) Enhanced tumor growth in brain dopamine-depleted mice following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treatment. J. Neuroimmunol., 60, 1–8. [DOI] [PubMed] [Google Scholar]
- 11. Bergquist, J., et al. (1998) Identification of catecholamines in the immune system by electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom., 12, 683–688. [DOI] [PubMed] [Google Scholar]
- 12. Allen, J.E., et al. (2016) Discovery and clinical introduction of first-in-class imipridone ONC201. Oncotarget, 7, 74380–74392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carlsson, A., et al. (1957) 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature, 180, 1200. [DOI] [PubMed] [Google Scholar]
- 14. Carlsson, A., et al. (1958) A fluorimetric method for the determination of dopamine (3-hydroxytyramine). Acta Physiol. Scand., 44, 293–298. [DOI] [PubMed] [Google Scholar]
- 15. Ehringer, H., et al. (1960) Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system. Klin Wochenschr., 38, 1236–1239. [DOI] [PubMed] [Google Scholar]
- 16. Birkmayer, W., et al. (1961) The l-3,4-dioxyphenylalanine (DOPA)-effect in Parkinson-akinesia. Wien. Klin. Wochenschr., 73, 787–788. [PubMed] [Google Scholar]
- 17. Cotzias, G.C. (1968) l-Dopa for Parkinsonism. N. Engl. J. Med., 278, 630. [DOI] [PubMed] [Google Scholar]
- 18. Bose, A., et al. (2018) Parkinson’s disease and melanoma: co-occurrence and mechanisms. J. Parkinsons Dis., 8, 385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ong, E.L., et al. (2014) Differential risks of cancer types in people with Parkinson’s disease: a national record-linkage study. Eur. J. Cancer, 50, 2456–2462. [DOI] [PubMed] [Google Scholar]
- 20. Inzelberg, R., et al. (2007) Are Parkinson disease patients protected from some but not all cancers? Neurology, 69, 1542–1550. [DOI] [PubMed] [Google Scholar]
- 21. Bajaj, A., et al. (2010) Parkinson’s disease and cancer risk: a systematic review and meta-analysis. Cancer Causes Control, 21, 697–707. [DOI] [PubMed] [Google Scholar]
- 22. Xie, X., et al. (2016) Risk of lung cancer in Parkinson’s disease. Oncotarget, 7, 77319–77325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Becker, C., et al. (2010) Cancer risk in association with Parkinson disease: a population-based study. Parkinsonism Relat. Disord., 16, 186–190. [DOI] [PubMed] [Google Scholar]
- 24. Minami, Y., et al. (2000) Mortality and cancer incidence in patients with Parkinson’s disease. J. Neurol., 247, 429–434. [DOI] [PubMed] [Google Scholar]
- 25. Lin, P.Y., et al. (2015) Association between Parkinson disease and risk of cancer in Taiwan. JAMA Oncol., 1, 633–640. [DOI] [PubMed] [Google Scholar]
- 26. Freedman, D.M., et al. (2016) Associations between cancer and Parkinson’s disease in U.S. elderly adults. Int. J. Epidemiol., 45, 741–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Olsen, J.H., et al. (2005) Atypical cancer pattern in patients with Parkinson’s disease. Br. J. Cancer, 92, 201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Olsen, J.H., et al. (2006) Malignant melanoma and other types of cancer preceding Parkinson disease. Epidemiology, 17, 582–587. [DOI] [PubMed] [Google Scholar]
- 29. Gao, X., et al. (2009) Family history of melanoma and Parkinson disease risk. Neurology, 73, 1286–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bertoni, J.M., et al. (2010) Increased melanoma risk in Parkinson disease: a prospective clinicopathological study. Arch. Neurol., 67, 347–352. [DOI] [PubMed] [Google Scholar]
- 31. Marks, R. (2000) Epidemiology of melanoma. Clin. Exp. Dermatol., 25, 459–463. [DOI] [PubMed] [Google Scholar]
- 32. Weiner, W.J., et al. (1993) Levodopa, melanoma, and Parkinson’s disease. Neurology, 43, 674–677. [DOI] [PubMed] [Google Scholar]
- 33. Elbaz, A., et al. (2005) Risk of cancer after the diagnosis of Parkinson’s disease: a historical cohort study. Mov. Disord., 20, 719–725. [DOI] [PubMed] [Google Scholar]
- 34. Dalvin, L.A., et al. (2017) Parkinson disease and melanoma: confirming and reexamining an association. Mayo Clin. Proc., 92, 1070–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bjorklund, A., et al. (2007) Fifty years of dopamine research. Trends Neurosci., 30, 185–187. [DOI] [PubMed] [Google Scholar]
- 36. Delay, J., et al. (1952) Therapeutic use in psychiatry of phenothiazine of central elective action (4560 RP). Ann. Med. Psychol. (Paris), 110, 112–117. [PubMed] [Google Scholar]
- 37. Cowden, R.C., et al. (1955) A preliminary note on the use of chlorpromazine with neuropsychiatric disorders. AMA Arch. Neurol. Psychiatry, 73, 700–701. [DOI] [PubMed] [Google Scholar]
- 38. Silvestri, S., et al. (2000) Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl.), 152, 174–180. [DOI] [PubMed] [Google Scholar]
- 39. Toda, M., et al. (2007) Dopamine hypothesis of schizophrenia: making sense of it all. Curr. Psychiatry Rep., 9, 329–336. [DOI] [PubMed] [Google Scholar]
- 40. van Rossum, J.M. (1966) The significance of dopamine-receptor blockade for the mechanism of action of neuroleptic drugs. Arch. Int. Pharmacodyn. Ther., 160, 492–494. [PubMed] [Google Scholar]
- 41. Eicke, W.J. (1973) Favorable course in carcinoma patients due to additional phenothiazine therapy. Med. Klin., 68, 1015–1018. [PubMed] [Google Scholar]
- 42. Osterman, E. (1961) Has chlorpromazine a cytostatic effect? A few reflections in connection with a case and the modern literature. Nord. Psykiatr. Tidsskr., 15, 154–159. [DOI] [PubMed] [Google Scholar]
- 43. Hercbergs, A. (1988) Thioridazine: a radiation enhancer in advanced cervical cancer? Lancet, 2, 737. [DOI] [PubMed] [Google Scholar]
- 44. Ettigi, P., et al. (1973) Prolactin, phenothiazines, admission to mental hospital, and carcinoma of the breast. Lancet, 2, 266–267. [DOI] [PubMed] [Google Scholar]
- 45. Mortensen, P.B. (1989) The incidence of cancer in schizophrenic patients. J. Epidemiol. Community Health, 43, 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lin, C.Y., et al. (2013) Inverse association between cancer risks and age in schizophrenic patients: a 12-year nationwide cohort study. Cancer Sci., 104, 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhuo, C., et al. (2018) Association of schizophrenia with the risk of breast cancer incidence: a meta-analysis. JAMA Psychiatry, 75, 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Weissenrieder, J.S., et al. (2019) Cancer and the dopamine D2 receptor: a pharmacological perspective. J. Pharmacol. Exp. Ther., 370, 111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gulbinat, W., et al. (1992) Cancer incidence of schizophrenic patients. Results of record linkage studies in three countries. Br. J. Psychiatry Suppl., (18), 75–83. [PubMed] [Google Scholar]
- 50. Gemignani, F., et al. (2005) Polymorphisms of the dopamine receptor gene DRD2 and colorectal cancer risk. Cancer Epidemiol. Biomarkers Prev., 14, 1633–1638. [DOI] [PubMed] [Google Scholar]
- 51. Murphy, G., et al. (2009) Dopamine D2 receptor polymorphisms and adenoma recurrence in the Polyp Prevention Trial. Int. J. Cancer, 124, 2148–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Duan, J., et al. (2003) Synonymous mutations in the human dopamine receptor D2 (DRD2) affect mRNA stability and synthesis of the receptor. Hum. Mol. Genet., 12, 205–216. [DOI] [PubMed] [Google Scholar]
- 53. Ritchie, T., et al. (2003) Association of seven polymorphisms of the D2 dopamine receptor gene with brain receptor-binding characteristics. Neurochem. Res., 28, 73–82. [DOI] [PubMed] [Google Scholar]
- 54. Pohjalainen, T., et al. (1998) The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Mol. Psychiatry, 3, 256–260. [DOI] [PubMed] [Google Scholar]
- 55. Basu, S., et al. (1999) Decreased dopamine receptor expression and its second-messenger cAMP in malignant human colon tissue. Dig. Dis. Sci., 44, 916–921. [DOI] [PubMed] [Google Scholar]
- 56. Basu, S., et al. (1997) Alteration of dopamine D2 receptors in human malignant stomach tissue. Dig. Dis. Sci., 42, 1260–1264. [DOI] [PubMed] [Google Scholar]
- 57. Robles, A.I., et al. (2014) A DRD1 polymorphism predisposes to lung cancer among those exposed to secondhand smoke during childhood. Cancer Prev. Res. (Phila)., 7, 1210–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sangrajrang, S., et al. (2010) Genetic polymorphisms in folate and alcohol metabolism and breast cancer risk: a case–control study in Thai women. Breast Cancer Res. Treat., 123, 885–893. [DOI] [PubMed] [Google Scholar]
- 59. Sun, Y., et al. (2015) Cancer mortality in people treated with antidepressants before cancer diagnosis: a population based cohort study. PLoS One, 10, e0138134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zingone, A., et al. (2017) Relationship between anti-depressant use and lung cancer survival. Cancer Treat Res. Commun., 10, 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Usiello, A., et al. (2000) Distinct functions of the two isoforms of dopamine D2 receptors. Nature, 408, 199–203. [DOI] [PubMed] [Google Scholar]
- 62. Radl, D., et al. (2013) Each individual isoform of the dopamine D2 receptor protects from lactotroph hyperplasia. Mol. Endocrinol., 27, 953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Roof, A.K., et al. (2018) The balance of PI3K and ERK signaling is dysregulated in prolactinoma and modulated by dopamine. Endocrinology, 159, 2421–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kojima, H., et al. (1999) Dopamine D1 receptor gene polymorphism and schizophrenia in Japan. Am. J. Med. Genet., 88, 116–119. [DOI] [PubMed] [Google Scholar]
- 65. Sato, M., et al. (2000) Dopamine D1 receptor gene polymorphism is associated with essential hypertension. Hypertension, 36, 183–186. [DOI] [PubMed] [Google Scholar]
- 66. Staessen, J.A., et al. (2008) Blood pressure and renal sodium handling in relation to genetic variation in the DRD1 promoter and GRK4. Hypertension, 51, 1643–1650. [DOI] [PubMed] [Google Scholar]
- 67. Pan, Y., et al. (2014) Association of dopamine D1 receptor gene polymorphism with schizophrenia: a meta-analysis. Neuropsychiatr. Dis. Treat., 10, 1133–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wick, M.M., et al. (1978) Enhancement of l-DOPA incorporation into melanoma by DOPA decarboxylase inhibition. J. Invest. Dermatol., 70, 358–360. [DOI] [PubMed] [Google Scholar]
- 69. Csatary, L.K. (1972) Chlorpromazines and cancer. Lancet, 2, 338–339. [DOI] [PubMed] [Google Scholar]
- 70. Pulkoski-Gross, A., et al. (2015) Repurposing the antipsychotic trifluoperazine as an antimetastasis agent. Mol. Pharmacol., 87, 501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Marques, L.O., et al. (2004) Trifluoperazine for schizophrenia. Cochrane Database Syst. Rev., (1), CD003545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Feng, Z., et al. (2018) The antipsychotic agent trifluoperazine hydrochloride suppresses triple-negative breast cancer tumor growth and brain metastasis by inducing G0/G1 arrest and apoptosis. Cell Death Dis., 9, 1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fancy, R.M., et al. (2018) Calmodulin antagonist enhances DR5-mediated apoptotic signaling in TRA-8 resistant triple negative breast cancer cells. J. Cell. Biochem., 119, 6216–6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yuan, K., et al. (2015) Calmodulin antagonists promote TRA-8 therapy of resistant pancreatic cancer. Oncotarget, 6, 25308–25319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kang, S., et al. (2017) Trifluoperazine, a well-known antipsychotic, inhibits glioblastoma invasion by binding to calmodulin and disinhibiting calcium release channel IP3R. Mol. Cancer Ther., 16, 217–227. [DOI] [PubMed] [Google Scholar]
- 76. Xia, Y., et al. (2019) Antipsychotic drug trifluoperazine suppresses colorectal cancer by inducing G0/G1 arrest and apoptosis. Front. Pharmacol., 10, 1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jiang, J., et al. (2017) Trifluoperazine activates FOXO1-related signals to inhibit tumor growth in hepatocellular carcinoma. DNA Cell Biol., 36, 813–821. [DOI] [PubMed] [Google Scholar]
- 78. Bhat, K., et al. (2020) The dopamine receptor antagonist trifluoperazine prevents phenotype conversion and improves survival in mouse models of glioblastoma. Proc. Natl Acad. Sci. USA, 117, 11085–11096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jandaghi, P., et al. (2016) Expression of DRD2 is increased in human pancreatic ductal adenocarcinoma and inhibitors slow tumor growth in mice. Gastroenterology, 151, 1218–1231. [DOI] [PubMed] [Google Scholar]
- 80. Kline, C.L.B., et al. (2018) Role of dopamine receptors in the anticancer activity of ONC201. Neoplasia, 20, 80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Li, J., et al. (2014) Genome-wide shRNA screen revealed integrated mitogenic signaling between dopamine receptor D2 (DRD2) and epidermal growth factor receptor (EGFR) in glioblastoma. Oncotarget, 5, 882–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Basu, S., et al. (2000) Role of dopamine in malignant tumor growth. Endocrine, 12, 237–241. [DOI] [PubMed] [Google Scholar]
- 83. Roy, S., et al. (2017) Activation of D2 dopamine receptors in CD133+ cancer stem cells in non-small cell lung carcinoma inhibits proliferation, clonogenic ability, and invasiveness of these cells. J. Biol. Chem., 292, 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Basu, S., et al. (2001) The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat. Med., 7, 569–574. [DOI] [PubMed] [Google Scholar]
- 85. Chakroborty, D., et al. (2004) Depleted dopamine in gastric cancer tissues: dopamine treatment retards growth of gastric cancer by inhibiting angiogenesis. Clin. Cancer Res., 10, 4349–4356. [DOI] [PubMed] [Google Scholar]
- 86. Lyden, D., et al. (2001) Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat. Med., 7, 1194–1201. [DOI] [PubMed] [Google Scholar]
- 87. Dvorak, H.F. (2005) Angiogenesis: update 2005. J. Thromb. Haemost., 3, 1835–1842. [DOI] [PubMed] [Google Scholar]
- 88. Chakroborty, D., et al. (2008) Dopamine regulates endothelial progenitor cell mobilization from mouse bone marrow in tumor vascularization. J. Clin. Invest., 118, 1380–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sarkar, C., et al. (2015) Dopamine is a safe antiangiogenic drug which can also prevent 5-fluorouracil induced neutropenia. Int. J. Cancer, 137, 744–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dolma, S., et al. (2016) Inhibition of dopamine receptor D4 impedes autophagic flux, proliferation, and survival of glioblastoma stem cells. Cancer Cell., 29, 859–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Haak, A.J., et al. (2019) Selective YAP/TAZ inhibition in fibroblasts via dopamine receptor D1 agonism reverses fibrosis. Sci. Transl. Med., 11. doi: 10.1126/scitranslmed.aau6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sachlos, E., et al. (2012) Identification of drugs including a dopamine receptor antagonist that selectively target cancer stem cells. Cell, 149, 1284–1297. [DOI] [PubMed] [Google Scholar]
- 93. Hussein, N., et al. (2018) Cariprazine, a dopamine D2/D3 receptor partial agonist, modulates ABCG2-mediated multidrug resistance in cancer. Cancers (Basel), 10, 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Calabrese, F., et al. (2020) The role of dopamine D3 receptors in the mechanism of action of cariprazine. CNS Spectr., 25, 343–351. [DOI] [PubMed] [Google Scholar]
- 95. Melmed, S., et al. (2011) Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab., 96, 273–288. [DOI] [PubMed] [Google Scholar]
- 96. Auriemma, R.S., et al. (2019) Dopamine agonists: from the 1970s to today. Neuroendocrinology, 109, 34–41. [DOI] [PubMed] [Google Scholar]
- 97. Albert, P.R., et al. (1990) Coupling of a cloned rat dopamine-D2 receptor to inhibition of adenylyl cyclase and prolactin secretion. J. Biol. Chem., 265, 2098–2104. [PubMed] [Google Scholar]
- 98. Keech, C.A., et al. (1992) Cyclic adenosine 3ʹ,5ʹ-monophosphate activation of the rat prolactin promoter is restricted to the pituitary-specific cell type. Mol. Endocrinol., 6, 2059–2070. [DOI] [PubMed] [Google Scholar]
- 99. Lew, A.M., et al. (1994) G(i) alpha 2- and G(o) alpha-mediated signaling in the Pit-1-dependent inhibition of the prolactin gene promoter. Control of transcription by dopamine D2 receptors. J. Biol. Chem., 269, 12007–12013. [PubMed] [Google Scholar]
- 100. Mouton, F., et al. (2008) TSH-secreting adenoma improved with cabergoline. Ann. Endocrinol. (Paris), 69, 244–248. [DOI] [PubMed] [Google Scholar]
- 101. Costa, R., et al. (2017) A pilot study of cabergoline for the treatment of metastatic breast cancer. Breast Cancer Res. Treat., 165, 585–592. [DOI] [PubMed] [Google Scholar]
- 102. Batista, R.L., et al. (2019) Cabergoline in the management of residual nonfunctioning pituitary adenoma: a single-center, open-label, 2-year randomized clinical trial. Am. J. Clin. Oncol., 42, 221–227. [DOI] [PubMed] [Google Scholar]
- 103. Calne, D.B., et al. (1974) Treatment of parkinsonism with bromocriptine. Lancet, 2, 1355–1356. [DOI] [PubMed] [Google Scholar]
- 104. Sachdev, Y., et al. (1975) Bromocriptine therapy in acromegaly. Lancet, 2, 1164–1168. [DOI] [PubMed] [Google Scholar]
- 105. Chamarthi, B., et al. (2015) Timed bromocriptine-QR therapy reduces progression of cardiovascular disease and dysglycemia in subjects with well-controlled type 2 diabetes mellitus. J. Diabetes Res., 2015, 157698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Triantafilo, N., et al. (2016) Cabergoline or bromocriptine for prolactinoma? Medwave, 16, e6545. [DOI] [PubMed] [Google Scholar]
- 107. Ishibashi, M., et al. (1994) Inhibition of growth of human small cell lung cancer by bromocriptine. Cancer Res., 54, 3442–3446. [PubMed] [Google Scholar]
- 108. Liberante, F.G., et al. (2016) Identification and validation of the dopamine agonist bromocriptine as a novel therapy for high-risk myelodysplastic syndromes and secondary acute myeloid leukemia. Oncotarget, 7, 6609–6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Lara-Castillo, M.C., et al. (2016) Repositioning of bromocriptine for treatment of acute myeloid leukemia. J. Transl. Med., 14, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Yang, Y., et al. (2018) Repositioning dopamine D2 receptor agonist bromocriptine to enhance docetaxel chemotherapy and treat bone metastatic prostate cancer. Mol. Cancer Ther., 17, 1859–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Jacobi, G.H., et al. (1978) Testosterone metabolism in patients with advanced carcinoma of the prostate: a comparative in vivo study of the effects of oestrogen and antiprolactin. Urol. Res., 6, 159–165. [DOI] [PubMed] [Google Scholar]
- 112. Jacobi, G.H., et al. (1979) Bromocriptine for palliation of advanced prostatic carcinoma. Experimental and clinical profile of a drug (author’s’ transl). Urol. Int., 34, 266–290. [DOI] [PubMed] [Google Scholar]
- 113. Horti, J., et al. (1998) A phase II study of bromocriptine in patients with androgen-independent prostate cancer. Oncol. Rep., 5, 893–896. [DOI] [PubMed] [Google Scholar]
- 114. Rho, S.B., et al. (2011) A gene signature-based approach identifies thioridazine as an inhibitor of phosphatidylinositol-3ʹ-kinase (PI3K)/AKT pathway in ovarian cancer cells. Gynecol. Oncol., 120, 121–127. [DOI] [PubMed] [Google Scholar]
- 115. Sudo, M., et al. (2015) Short-hairpin RNA library: identification of therapeutic partners for gefitinib-resistant non-small cell lung cancer. Oncotarget, 6, 814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Aslostovar, L., et al. (2018) A phase 1 trial evaluating thioridazine in combination with cytarabine in patients with acute myeloid leukemia. Blood Adv., 2, 1935–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Menkes, D.B., et al. (2002) Cardiotoxicity and prescription of thioridazine in New Zealand. Aust. NZ J. Psychiatry, 36, 492–498. [DOI] [PubMed] [Google Scholar]
- 118. Prabhu, V.V., et al. (2015) Small-molecule ONC201/TIC10 targets chemotherapy-resistant colorectal cancer stem-like cells in an Akt/Foxo3a/TRAIL-dependent manner. Cancer Res., 75, 1423–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Tu, Y.S., et al. (2017) The imipridone ONC201 induces apoptosis and overcomes chemotherapy resistance by up-regulation of Bim in multiple myeloma. Neoplasia, 19, 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Greer, Y.E., et al. (2018) ONC201 kills breast cancer cells in vitro by targeting mitochondria. Oncotarget, 9, 18454–18479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Wang, S., et al. (2019) The direct molecular target for imipridone ONC201 is finally established. Cancer Cell., 35, 707–708. [DOI] [PubMed] [Google Scholar]
- 122. Allen, J.E., et al. (2015) Identification of TRAIL-inducing compounds highlights small molecule ONC201/TIC10 as a unique anti-cancer agent that activates the TRAIL pathway. Mol. Cancer, 14, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Prabhu, V.V., et al. (2020) ONC201 and imipridones: anti-cancer compounds with clinical efficacy. Neoplasia, 22, 725–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Allen, J.E., et al. (2013) Dual inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci. Transl. Med., 5, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Madhukar, N.S., et al. (2019) A Bayesian machine learning approach for drug target identification using diverse data types. Nat. Commun., 10, 5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Prabhu, V.V., et al. (2019) Dopamine receptor D5 is a modulator of tumor response to dopamine receptor D2 antagonism. Clin. Cancer Res., 25, 2305–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ishizawa, J., et al. (2016) ATF4 induction through an atypical integrated stress response to ONC201 triggers p53-independent apoptosis in hematological malignancies. Sci. Signal., 9, ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kline, C.L., et al. (2016) ONC201 kills solid tumor cells by triggering an integrated stress response dependent on ATF4 activation by specific eIF2alpha kinases. Sci. Signal., 9, ra18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kauppila, A., et al. (1981) Metoclopramide increases prolactin release and milk secretion in puerperium without stimulating the secretion of thyrotropin and thyroid hormones. J. Clin. Endocrinol. Metab., 52, 436–439. [DOI] [PubMed] [Google Scholar]
- 130. Peuskens, J., et al. (2014) The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs, 28, 421–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Arrillaga-Romany, I., et al. (2017) A phase 2 study of the first imipridone ONC201, a selective DRD2 antagonist for oncology, administered every three weeks in recurrent glioblastoma. Oncotarget, 8, 79298–79304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Di, Y.Z., et al. (2019) Role of the brain–gut axis in gastrointestinal cancer. World J. Clin. Cases, 7, 1554–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kou, X., et al. (2014) Dopamine D(1)-like receptors suppress proliferation of vascular smooth muscle cell induced by insulin-like growth factor-1. Clin. Exp. Hypertens., 36, 140–147. [DOI] [PubMed] [Google Scholar]
- 134. Borcherding, D.C., et al. (2016) Expression and therapeutic targeting of dopamine receptor-1 (D1R) in breast cancer. Oncogene, 35, 3103–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Yan, Y., et al. (2020) Increased dopamine and its receptor dopamine receptor D1 promote tumor growth in human hepatocellular carcinoma. Cancer Commun. (Lond.), 40, 694–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Leng, Z.G., et al. (2017) Activation of DRD5 (dopamine receptor D5) inhibits tumor growth by autophagic cell death. Autophagy, 13, 1404–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Yamauchi, M., et al. (2018) The fibrotic tumor stroma. J. Clin. Invest., 128, 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Karampitsakos, T., et al. (2017) Lung cancer in patients with idiopathic pulmonary fibrosis. Pulm. Pharmacol. Ther., 45, 1–10. [DOI] [PubMed] [Google Scholar]
- 139. Guerra, L., et al. (2017) Stromal microenvironment in type VII collagen-deficient skin: the ground for squamous cell carcinoma development. Matrix Biol., 63, 1–10. [DOI] [PubMed] [Google Scholar]
- 140. Laklai, H., et al. (2016) Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat. Med., 22, 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Cheng, H.W., et al. (2015) Identification of thioridazine, an antipsychotic drug, as an antiglioblastoma and anticancer stem cell agent using public gene expression data. Cell Death Dis., 6, e1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Lee, S.M., et al. (2014) Dopamine D1 receptor signaling: does GalphaQ-phospholipase C actually play a role? J. Pharmacol. Exp. Ther., 351, 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Song, M., et al. (2019) AKT as a therapeutic target for cancer. Cancer Res., 79, 1019–1031. [DOI] [PubMed] [Google Scholar]
- 144. Cheng, Y., et al. (2017) Current development status of MEK inhibitors. Molecules, 22, 1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Felten, D.L., et al. (1988) Sympathetic noradrenergic innervation of immune organs. Brain Behav. Immun., 2, 293–300. [DOI] [PubMed] [Google Scholar]
- 146. Elenkov, I.J., et al. (2000) The sympathetic nerve—an integrative interface between two supersystems: the brain and the immune system. Pharmacol. Rev., 52, 595–638. [PubMed] [Google Scholar]
- 147. Straub, R.H. (2004) Complexity of the bi-directional neuroimmune junction in the spleen. Trends Pharmacol. Sci., 25, 640–646. [DOI] [PubMed] [Google Scholar]
- 148. Cosentino, M., et al. (2015) Sympathoadrenergic modulation of hematopoiesis: a review of available evidence and of therapeutic perspectives. Front. Cell. Neurosci., 9, 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.