Abstract
Background and objectives
Transthoracic echocardiography may potentially be useful to obtain a prompt, accurate and non-invasive estimation of cardiac output. We evaluated whether non-cardiologist intensivists may obtain accurate and reproducible cardiac output determination in hemodynamically unstable mechanically ventilated patients.
Methods
We studied 25 hemodynamically unstable mechanically ventilated intensive care unit patients with a pulmonary artery catheter in place. Cardiac output was calculated using the pulsed Doppler transthoracic echocardiography technique applied to the left ventricular outflow tract in apical 5 chamber view by two intensive care unit physicians who had received a basic Transthoracic Echocardiography training plus a specific training focused on Doppler, left ventricular outflow tract and velocity-time integral determination.
Results
Cardiac output assessment by transthoracic echocardiography was feasible in 20 out of 25 enrolled patients (80%) and showed an excellent inter-operator reproducibility (Pearson correlation test r = 0.987; Cohen's K = 0.840). Overall, the mean bias was 0.03 L.min−1, with limits of agreement −0.52 and +0.57 L.min−1. The concordance correlation coefficient (ρc) was 0.986 (95% IC 0.966–0.995) and 0.995 (95% IC 0.986–0.998) for physician 1 and 2, respectively. The value of accuracy (Cb) of COTTE measurement was 0.999 for both observers. The value of precision (ρ) of COTTE measurement was 0.986 and 0.995 for observer 1 and 2, respectively.
Conclusions
A specific training focused on Doppler and VTI determination added to the standard basic transthoracic echocardiography training allowed non-cardiologist intensive care unit physicians to achieve a quick, reproducible and accurate snapshot cardiac output assessment in the majority of mechanically ventilated intensive care unit patients.
Keywords: Cardiac output, Transthoracic echocardiography, Pulmonary artery catheter, Intensive Care Unit
Resumo
Justificativa e objetivos
A ecocardiografia transtorácica pode ser potencialmente útil para obter uma estimativa rápida, precisa e não invasiva do débito cardíaco. Avaliamos se os intensivistas não cardiologistas podem obter uma determinação precisa e reprodutível do débito cardíaco em pacientes mecanicamente ventilados e hemodinamicamente instáveis.
Métodos
Avaliamos 25 pacientes em unidade de terapia intensiva, mecanicamente ventilados, hemodinamicamente instáveis, com cateteres de artéria pulmonar posicionados. O débito cardíaco foi calculado usando a técnica de ecocardiografia transtorácica com Doppler pulsátil aplicada à via de saída do ventrículo esquerdo no corte apical (5-câmaras) por dois médicos intensivistas que receberam treinamento básico em ecocardiografia transtorácica e treinamento específico focado em Doppler, via de saída do ventrículo esquerdo e determinação da integral de tempo-velocidade.
Resultados
A avaliação do débito cardíaco pelo ecocardiograma transtorácico foi factível em 20 dos 25 pacientes inscritos (80%) e mostrou excelente reprodutibilidade entre operadores (teste de correlação de Pearson r = 0,987; K de Cohen = 0,840). No geral, o viés médio foi de 0,03 L.min−1, com limites de concordância de −0,52 e +0,57 L.min−1. O coeficiente de correlação de concordância (ρc) foi 0,986 (95% IC 0,966–0,995) e 0,995 (95% IC 0,986–0,998) para os médicos 1 e 2, respectivamente. O valor de precisão (Cb) da mensuração de COTTE foi de 0,999 para ambos os observadores. O valor de precisão (ρ) da mensuração de COTTE foi de 0,986 e 0,995 para os observadores 1 e 2, respectivamente.
Conclusões
Um treinamento específico focado na determinação do Doppler e VTI, adicionado ao treinamento padrão em ecocardiografia transtorácica básica, permitiu que médicos não cardiologistas da unidade de terapia intensiva obtivessem uma avaliação rápida, reprodutível e precisa do débito cardíaco instantâneo na maioria dos pacientes mecanicamente ventilados em unidade de terapia intensiva.
Palavras-chave: Débito cardíaco, Ecocardiografia transtorácica, Cateter de artéria pulmonar, Unidade de Terapia Intensiva
Introduction
Assessment of Cardiac Output (CO) may help managing patients with impaired hemodynamics in Emergency and Intensive Care Unit (ICU) and facilitate prompt and proper treatment.
The use of the pulmonary artery catheter (PAC) is, nonetheless, overall declining and advised only for the most complex and severe cases.1, 2, 3 Recent years have seen the increased availability of less-invasive devices providing, in association with other hemodynamic variables, surrogate CO estimates. Invasiveness, complexity, technical limitations and costs of these devices, however, have never been weighted against clinical benefit, and indications and proper timing of application remain unclear.1, 2, 3 Transthoracic Echocardiography (TTE) was proposed decades ago as a means to obtain a non-invasive snapshot determination of CO (COTTE).4 When performed by TTE experienced cardiologists, COTTE provided a reliable CO estimation in clinically stable patients with chronic heart failure, compared to CO assessed by PAC (COPAC).5 Some case series published in the mid-nineties indicated the potentials of the echocardiographic technique also in critically ill patients.6, 7 In spite of these positive initial reports, however, these investigations were neither followed by other studies nor by diffuse clinical application, likely because of the problematic attainment of immediate availability of TTE experienced cardiologists in the emergency and ICU settings.8 Recent work, however, shows that Emergency and ICU physicians can proficiently perform basic TTE examinations to qualitatively assess left ventricular function and volemic status and responsiveness following a relatively brief training in image acquisition and interpretation,8, 9 with the advantage of prompt TTE availability and possibility of repeated examinations in order to evaluate the response to therapeutic interventions.10
We therefore designed this study to evaluate whether non-cardiologist intensivists may obtain quite accurate and reproducible CO determination in hemodynamically unstable mechanically ventilated patients.
Materials and methods
The study was conducted in a 14 bed ICU of a University Hospital, in accordance with the principles outlined in the Declaration of Helsinki. Ethical approval for this study (Ethical Committee no. CE44/11) was provided by the Institutional Ethics Committee of Maggiore della Carità University Hospital. Written informed consent was obtained for each enrolled patient before inclusion in the study. We considered eligible any ICU patient ≥18 y.o. with the PAC already in place for clinical purposes. Patients were excluded (1) a priori, because of (a) arrhythmias or (b) known moderate or severe aortic valve disease, or (2) during TTE assessment, for (a) inadequate acoustic window, (b) detection of unknown moderate or severe aortic valve disease, (c) detection of unknown moderate or severe tricuspid valve regurgitation.
TTE was performed by two ICU physicians who had already received a basic training (TTE 3 hour course followed by 6 hour hands-on and by 50 tutored TTE evaluations) by an ultrasound expert cardiologist, focused on standard echocardiographic views and identification of relevant ventricular and valvular pathologic findings. Additionally, they underwent a specific training focused on continuous and pulsatile Doppler, Left Ventricular Outflow Tract (LVOT) and Velocity-Time Integral (VTI) determination (5 hour course followed by 6 hour hands-on). Before the study was initiated, both ICU physicians performed a minimum of 25 successfully tutored TTE evaluations of VTI and LVOT; accordingly, they both had completed their learning curve and were able to correctly assess LVOT and VTI as certified by the expert cardiac sonographer.
For each patient, COPAC was determined with a Swan-Ganz catheter (Edwards Lifesciences, Irvine, CA, USA) as the average of three consecutive thermodilution measurements (IntelliVue MX700, Philips, Netherlands) by the attending physician. The measurements were postponed when changes in hemodynamics occurred such to require intervention (i.e., administration of fluids or change in the vasoactive therapy). Immediately after COPAC assessment, the two investigators, blinded to each other and to the COPAC values, sequentially performed TTE with the portable device in use in our ICU (MyLab 30 CV, Esaote, Italy), according to a predetermined random sequence. Both COPAC and COTTE were measured with the patient in supine or semi-recumbent position. COTTE was obtained through the LVOT method, according to the technique originally proposed by Dubin et al.11 Briefly, the LVOT was measured in systole from the parasternal long axis view just below the insertion of the aortic cusps, and the area was then calculated according to the formula πr2 (Fig. 2A). Three measurements were averaged. The velocity of LVOT flow was measured by pulsed-wave Doppler from the apical 5 chamber view. The sample volume was positioned in the middle of the outflow tract immediately below the aortic cusps and the time velocity integrals, recorded over 5 consecutive cardiac cycles, were digitized using the leading edge convention (Fig. 2B). CO was then automatically calculated, according to the formula VTI × LVOT area × HR, where VTI is velocity-time integral, LVOT is left ventricular outflow tract cross-sectional area, and HR indicates the average of the instantaneous heart rate of 5 consecutive cardiac cycles, in the course of the expiratory phase. We assessed both inter-observer agreement and correlation between TTE and PAC. The Pearson correlation was used to evaluate the agreement of the COTTE values obtained by the two operators for each patient. A correlation coefficient >0.8 with p < 0.05 was considered to indicate adequate reproducibility. Moreover, Cohen's K was also calculated, as indicated when comparing the same measurement performed by two or more operators. We performed Bland and Altman analysis by plotting for each patient the difference between COPAC values and corresponding COTTE measurements individually obtained by the two ICU physicians.12 We further calculated the limits of agreement between the two techniques, as proposed by Critchley et al.13 According to the same authors, we assessed TTE agreement with the reference COPAC using the concordance correlation coefficient (ρc), which also allows determining accuracy, through the bias correction factor (Cb), and precision, through the Pearson correlation coefficient (ρ).13
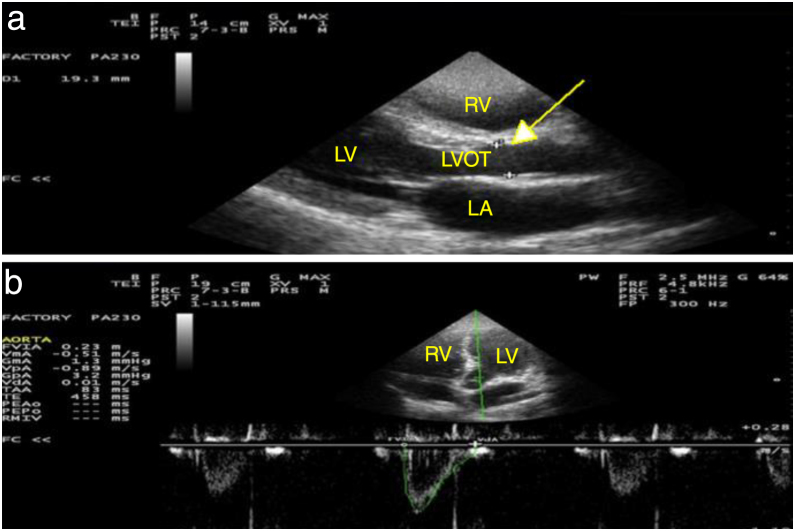
Figure 2.
Echocardiographic measurements from one representative patient. The figure shows: (A) measurement of LVOT diameter at aortic valve cusps through parasternal long-axis view (indicated by a yellow arrow) and (B) measurement of velocity-time integral using apical 5 chamber view (indicated as FVIA in our sonographer). RV, Right Ventricle; LV, Left Ventricle; LA, Left Atrium; LVOT, Left Ventricular Outflow Tract; FVIA, Flow Velocity Integral (aorta).
Results
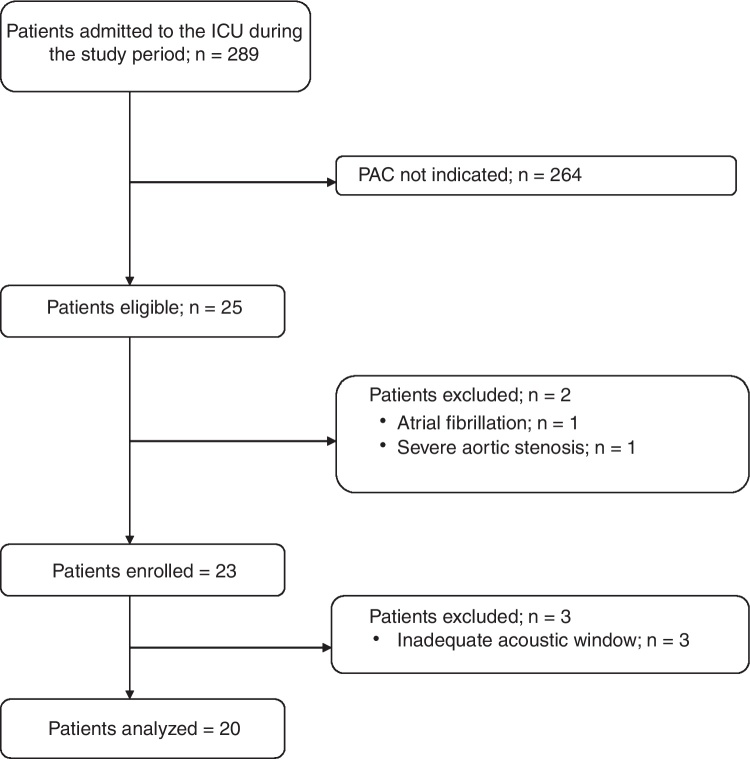
During the 4 month study period, 25 patients of 289 admitted to the ICU, met the inclusion criteria. Of these 25 patients, two were excluded a priori (one for high ventricular rate atrial fibrillation and one for known severe aortic stenosis), while three (one with partial left pneumothorax following thoracic surgery and two with severe chronic obstructive pulmonary disease) because of inadequate acoustic window, as stated by both operators. Therefore, 20 patients (80%) were included in the analysis (Fig. 1). All patients underwent volume-targeted controlled mechanical ventilation with Positive End-Expiratory Pressure (PEEP) ranging between 5 and 20 cm H2O, and received continuous sedative infusion. Inotropes and/or vasopressors were administered to all patients for treatment of hemodynamic instability associated with cardiogenic shock (5 patients), acute respiratory distress syndrome (7 patients) and septic shock (8 patients) (Table 1).
Figure 1.
Flow of patients in the study (ICU, Intensive Care Unit; PAC, Pulmonary Artery Catheter).
Table 1.
Patients’ characteristics at enrolment.
| Patient | Age (y) | M/F | BSA | Diagnosis | PEEP | Inotropes/vasopressors | Dose (mcg.kg−1.min−1) |
|---|---|---|---|---|---|---|---|
| 1 | 67 | M | 2.15 | ARDS | 15 | Dopamine | 7.0 |
| 2 | 53 | M | 1.97 | AMI | 5 | Epinephrine | 0.1 |
| 3 | 71 | M | 2.31 | Septic shock | 12 | Norepinephrine | 0.7 |
| 4 | 26 | F | 1.49 | Septic shock | 15 | Norepinephrine | 0.8 |
| 5 | 61 | M | 1.95 | AMI | 7 | Dopamine | 8.2 |
| 6 | 54 | F | 1.73 | PE | 5 | Epinephrine | 0.1 |
| 7 | 69 | M | 1.78 | AMI | 8 | Dobutamine | 7.7 |
| 8 | 70 | M | 2.26 | ARDS | 20 | Norepinephrine | 1.1 |
| 9 | 29 | F | 1.60 | Septic shock | 15 | Norepinephrine | 1.0 |
| 10 | 74 | M | 2.09 | AMI | 7 | Dopamine | 8.0 |
| 11 | 72 | F | 1.78 | ARDS | 12 | Dopamine | 9.0 |
| 12 | 78 | M | 2.07 | Septic shock | 11 | Norepinephrine | 1.2 |
| 13 | 19 | M | 1.68 | ARDS | 15 | Dopamine | 9.0 |
| 14 | 73 | M | 2.06 | Septic shock | 18 | Norepinephrine | 0.5 |
| 15 | 74 | M | 1.97 | ARDS | 12 | Norepinephrine | 0.7 |
| 16 | 64 | M | 2.21 | Septic shock | 10 | Norepinephrine | 0.8 |
| 17 | 41 | M | 2.04 | Septic shock | 10 | Norepinephrine | 1.2 |
| 18 | 75 | F | 1.69 | Septic shock | 5 | Norepinephrine | 0.5 |
| 19 | 67 | M | 1.94 | ARDS | 15 | Norepinephrine | 0.4 |
| 20 | 41 | M | 2.01 | ARDS | 14 | Norepinephrine | 0.8 |
BSA, Body Surface Area; PEEP, Positive End-Expiratory Pressure; ARDS, Acute Respiratory Distress Syndrome; AMI, Acute Myocardial Infarction; PE, Pulmonary Embolism.
Table 2 displays for each patient, from left to right, the individual COTTE determinations by each operator, the average of these two values, and the corresponding COPAC measurements. The r-value of the correlation between COTTE determinations by the two operators was 0.98 and the Cohen's K 0.84, indicating good inter-observer reproducibility.
Table 2.
Values of Cardiac Output determined by Transthoracic Echocardiography and by Pulmonary Artery Catheter.
| Patient | O1-COTTE (L.min−1) | O2-COTTE (L.min−1) | O1–2avg-COTTE (L.min−1) | COPAC (L.min−1) |
|---|---|---|---|---|
| 1 | 7.57 | 6.76 | 7.16 | 6.65 |
| 2 | 4.40 | 4.51 | 4.45 | 4.47 |
| 3 | 8.09 | 8.28 | 8.18 | 8.18 |
| 4 | 4.89 | 5.34 | 5.11 | 5.18 |
| 5 | 3.43 | 3.58 | 3.50 | 3.79 |
| 6 | 2.89 | 2.90 | 2.89 | 2.77 |
| 7 | 3.61 | 3.49 | 3.55 | 3.55 |
| 8 | 8.54 | 8.35 | 8.44 | 8.12 |
| 9 | 7.15 | 7.15 | 7.15 | 7.32 |
| 10 | 5.62 | 5.35 | 5.48 | 5.25 |
| 11 | 4.12 | 4.12 | 4.12 | 4.58 |
| 12 | 8.90 | 8.81 | 8.85 | 8.89 |
| 13 | 5.60 | 5.61 | 5.60 | 5.42 |
| 14 | 7.75 | 8.40 | 8.07 | 8.12 |
| 15 | 4.40 | 4.64 | 4.52 | 4.42 |
| 16 | 8.59 | 9.28 | 8.93 | 8.98 |
| 17 | 9.05 | 8.85 | 8.95 | 8.77 |
| 18 | 7.84 | 8.03 | 7.93 | 8.22 |
| 19 | 6.31 | 6.58 | 6.44 | 6.39 |
| 20 | 4.27 | 4.61 | 4.44 | 4.26 |
| Mean ± SD | 6.15 ± 2.00 | 6.23 ± 2.01 | 6.18 ± 1.99 | 6.14 ± 2.02 |
O1-COTTE, Cardiac Output determined by Transthoracic Echocardiography by observer 1; O2-COTTE, Cardiac Output determined by Transthoracic Echocardiography by observer 2; O1–2avg-COTTE, average of O1-COTTE and O2-COTTE values; COPAC, Cardiac Output determined by the Pulmonary Artery Catheter; SD, Standard Deviation.
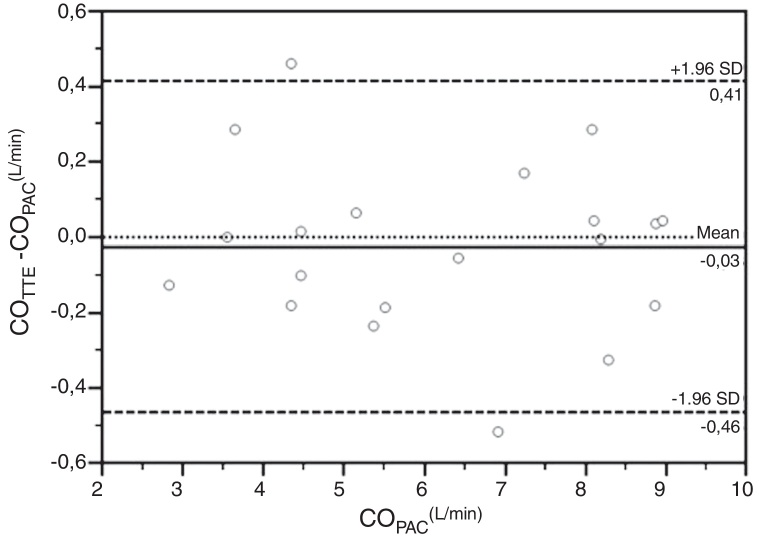
Fig. 3 depicts Bland and Altman plot of the differences between COPAC and the COTTE values obtained by the two operators. Overall, the mean bias was 0.03 L.min−1, with limits of agreement −0.52 and +0.57 L.min−1. The concordance correlation coefficient (ρc) was 0.986 (95% IC 0.966–0.995) and 0.995 (95% IC 0.986–0.998) for the observer 1 and 2, respectively. The value of accuracy (Cb) of COTTE measurement was 0.999 for both observers. The value of precision (ρ) of COTTE measurement was 0.986 and 0.995 for observer 1 and observer 2, respectively. Averaging the COTTE values obtained by the two operators, the predicted limit of agreement was 11%, definitely below the 30% threshold identified by Critchley et al. to define acceptable agreement.13
Figure 3.
Bland and Altman plot of the differences between COTTE and COPAC. COTTE values are obtained averaging the determinations of the two observers. COTTE, Cardiac Output determined with Transthoracic Echocardiography; COPAC, Cardiac Output determined with the Pulmonary Artery Catheter; SD, Standard Deviation.
Discussion
The major finding of our study is that non-cardiologist ICU physicians who received a relatively brief training on Doppler and VTI determination in addition to the standard training of basic TTE were able to accurately estimate CO in mechanically ventilated ICU patients.
The use of PAC is currently markedly declining. Specific indications for PAC monitoring in ICU remain the diagnosis and treatment of acute right ventricular failure and pulmonary hypertension, weaning failure of cardiac origin1 and post cardiac surgery.14 PAC also remains indicated for ICU patients with severe heart failure, requiring inotropic, vasopressor, and/or vasodilator therapy.3 Less complex ICU patients without any of the aforementioned indications for PAC monitoring, however, may also experience hemodynamic instability. In these patients, adding a non-invasive snapshot CO assessment may be valuable to properly choose between fluids and inotropic or vasoactive agents. Worth remarking, TTE gives information that is not limited to CO assessment and may be associated to invasive monitoring also in the most severe patients.
TTE has gained ground in ICU and is nowadays considered a valuable tool to assess left ventricular function even when performed by intensivists with a relatively brief training; furthermore, some consider TTE a first-line approach for initial evaluation of hemodynamic failure in ICU and to assess fluid responsiveness.15 In keeping with some case series published almost two decades ago reporting TTE to accurately estimate CO when performed by experienced cardiologists,5 our results indicate that TTE offers the possibility to achieve satisfactory CO estimation in mechanically ventilated patients for whom the use of PAC or other forms of less-invasive monitoring is neither feasible nor strictly indicated. Worth remarking, our results were obtained by intensivists after a relatively brief training, extending to mechanically ventilated ICU patients the findings of a previous study where COTTE was determined by two Emergency physicians, who had previously received a 20 hour training by an expert cardiac sonographer, in non-critical patients.16 While in this prior study the COTTE values determined by the two Emergency physicians were compared with those obtained by two certified cardiac sonographers,16 in our study we compare COTTE directly with COPAC.
Though an adequate training is considered essential for a successful TTE-based clinical decision making,10 there is little agreement on the number of cardiac ultrasound examinations to be performed by ICU physicians before achieving an appropriate training. A core curriculum and necessary training elements for ICU physicians have been proposed by Mazraeshahi et al.,17 who consider 10–20 successful interrogations adequate to achieve procedural competency on most of the aortic pathologies.17 In the present study, the two ICU physicians involved, already trained and experienced in basic TTE, received a specific training for LVOT and VTI determination including a minimum of 25 tutored successful evaluations. In keeping with previous work, our results indicate this quite limited specific training to be adequate to perform a focused goal-directed TTE, such as quantitative CO evaluation.16
Although COTTE determination has been successfully applied in non-ICU patients with chronic atrial fibrillation,5 we preferred to exclude patients with arrhythmias to avoid interference due to remarkable variations between consecutive systolic stroke volumes. We also excluded patients with aortic valvular diseases which may impair the quantitative analysis of Doppler velocity consequent to changes in the spatial profile of blood flow instantaneous velocity. In addition to arrhythmias, making arduous to obtain a representative mean VTI, and aortic valve disease, hampering the quantitative analysis of Doppler velocity, the applicability of TTE to estimate CO in critical patients can be restricted by difficult achievement of adequate acoustic window, consequent to supine position, mechanical ventilation and lung and/or chest wall alterations.
Moreover, the elliptical shape of LVOT could lead to inaccurate LVOT area calculation between two observers due to different diameter measurements. However, in our study the inter-observer agreement and the correlation between COTTE and COPAC were very high.
Finally, we attempted to avert the risk of variations in stroke volume secondary to cardiopulmonary interactions by selecting the 5 cardiac cycles during the ventilator expiratory phase. We cannot exclude, however, that some of the averaged cardiac cycles actually occurred during the insufflation phase. We share with all the studies using this technique this possible limitation, which is indeed more likely to occur in patients with spontaneous breathing activity, where the respiratory rate cannot be controlled. Notwithstanding these technical limitations, COTTE was feasible in the vast majority of mechanically ventilated patients, as both observers were able to determine it in 80% of the patients. Remarkable, COTTE was feasible also in 5 patients with PEEP ≥ 15 cm H2O.
Our data confirm those of recent reports. In 55 ICU patients with shock receiving mechanical ventilation, Bergenzaun et al. obtained acceptable TTE images in more than 90% of the examinations.18 Amiel et al. in 94 ICU patients, 63% of whom were mechanically ventilated, found left ventricle ejection fraction impossible to determine in 10 individuals only.19 Dinh et al. were able to determine LVOT, VTI, and CO in 97 of 100 non-critically ill patients in an Emergency Department.16
It might be argued that the importance of our study is limited by the relatively small number of patients. It should be considered, however, that for the purposes of our investigation a sample of 14 patients would be sufficient to obtain a correlation coefficient of 0.8 with a power of 0.95 and an alpha error of 0.05. Since we included in the data analysis 20 patients and obtained much higher r-values, the risk of Type II error is very unlikely.
Even though for the purpose of this study we included only ICU patients with severely unstable hemodynamics requiring invasive monitoring, we believe TTE should not be considered as a replacement for PAC or other forms of monitoring in the most severe patients, but rather as a means to extend CO assessment to hypotensive patients for whom hemodynamic monitoring is unfeasible, unavailable, not strictly indicated or temporarily contraindicated. Importantly, as hemodynamic monitoring does not guarantee per se improved outcome, unless part of an appropriate therapeutic plan, COTTE should also be utilized within a specific scheme of treatment for hemodynamic instability.20
Summary
A specific training focused on Doppler and VTI determination added to the standard training of basic TTE allowed non-cardiologist ICU physicians to achieve a quick, reproducible and accurate snapshot CO assessment in the majority of mechanically ventilated ICU patients.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Vincent J.L., Pinsky M.R., Sprung C.L., et al. The pulmonary artery catheter: in medio virtus. Crit Care Med. 2008;36:3093–3096. doi: 10.1097/CCM.0b013e31818c10c7. [DOI] [PubMed] [Google Scholar]
- 2.Rajaram S.S., Desai N.K., Kalra A., et al. Pulmonary artery catheters for adult patients in intensive care. Cochrane Database Syst Rev. 2013;2:CD003408. doi: 10.1002/14651858.CD003408.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chatterjee K. The Swan-Ganz catheters: past, present, and future. A viewpoint. Circulation. 2009;119:147–152. doi: 10.1161/CIRCULATIONAHA.108.811141. [DOI] [PubMed] [Google Scholar]
- 4.Coats A.J. Doppler ultrasonic measurement of cardiac output: reproducibility and validation. Eur Heart J. 1990;11(Suppl. I):49–61. doi: 10.1093/eurheartj/11.suppl_i.49. [DOI] [PubMed] [Google Scholar]
- 5.Gola A., Pozzoli M., Capomolla S., et al. Comparison of Doppler echocardiography with thermodilution for assessing cardiac output in advanced congestive heart failure. Am J Cardiol. 1996;78:708–712. doi: 10.1016/s0002-9149(96)00406-7. [DOI] [PubMed] [Google Scholar]
- 6.Dabaghi S.F., Rokey R., Rivera J.M., et al. Comparison of echocardiographic assessment of cardiac hemodynamics in the intensive care unit with right-sided cardiac catheterization. Am J Cardiol. 1995;76:392–395. doi: 10.1016/s0002-9149(99)80107-6. [DOI] [PubMed] [Google Scholar]
- 7.Feinberg M.S., Hopkins W.E., Davila-Roman V.G., et al. Multiplane transesophageal echocardiographic doppler imaging accurately determines cardiac output measurements in critically ill patients. Chest. 1995;107:769–773. doi: 10.1378/chest.107.3.769. [DOI] [PubMed] [Google Scholar]
- 8.Melamed R., Sprenkle M.D., Ulstad V.K., et al. Assessment of left ventricular function by intensivists using hand-held echocardiography. Chest. 2009;135:1416–1420. doi: 10.1378/chest.08-2440. [DOI] [PubMed] [Google Scholar]
- 9.Jones A.E., Tayal V.S., Sullivan D.M., et al. Randomized, controlled trial of immediate versus delayed goal-directed ultrasound to identify the cause of nontraumatic hypotension in emergency department patients. Crit Care Med. 2004;32:1703–1708. doi: 10.1097/01.ccm.0000133017.34137.82. [DOI] [PubMed] [Google Scholar]
- 10.Marik P.E., Mayo P. Certification and training in critical care ultrasound. Intensive Care Med. 2008;34:215–217. doi: 10.1007/s00134-007-0924-4. [DOI] [PubMed] [Google Scholar]
- 11.Dubin J., Wallerson D.C., Cody R.J., et al. Comparative accuracy of Doppler echocardiographic methods for clinical stroke volume determination. Am Heart J. 1990;120:116–123. doi: 10.1016/0002-8703(90)90168-w. [DOI] [PubMed] [Google Scholar]
- 12.Bland J.M., Altman D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 13.Critchley L.A., Critchley J.A. A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput. 1999;15:85–91. doi: 10.1023/a:1009982611386. [DOI] [PubMed] [Google Scholar]
- 14.Jacka M.J., Cohen M.M., To T., et al. The appropriateness of the pulmonary artery catheter in cardiovascular surgery. Can J Anaesth. 2002;49:276–282. doi: 10.1007/BF03020527. [DOI] [PubMed] [Google Scholar]
- 15.Vieillard-Baron A., Slama M., Cholley B., et al. Echocardiography in the intensive care unit: from evolution to revolution? Intensive Care Med. 2008;34:243–249. doi: 10.1007/s00134-007-0923-5. [DOI] [PubMed] [Google Scholar]
- 16.Dinh V.A., Ko H.S., Rao R., et al. Measuring cardiac index with a focused cardiac ultrasound examination in the ED. Am J Emerg Med. 2012;30:1845–1851. doi: 10.1016/j.ajem.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 17.Mazraeshahi R.M., Farmer J.C., Porembka D.T. A suggested curriculum in echocardiography for critical care physicians. Crit Care Med. 2007;35:S431–S433. doi: 10.1097/01.CCM.0000270280.65365.AA. [DOI] [PubMed] [Google Scholar]
- 18.Bergenzaun L., Gudmundsson P., Ohlin H., et al. Assessing left ventricular systolic function in shock: evaluation of echocardiographic parameters in intensive care. Crit Care. 2011;15:R200. doi: 10.1186/cc10368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amiel J.B., Grumann A., Lheritier G., et al. Assessment of left ventricular ejection fraction using an ultrasonic stethoscope in critically ill patients. Crit Care. 2012;16:R29. doi: 10.1186/cc11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinsky M.R., Vincent J.L. Let us use the pulmonary artery catheter correctly and only when we need it. Crit c Care Med. 2005;33:1119–1122. doi: 10.1097/01.ccm.0000163238.64905.56. [DOI] [PubMed] [Google Scholar]