Abstract
The proceeding pandemic of coronavirus disease 2019 is the latest global challenge. Like most other infectious diseases, inflammation, oxidative stress, and immune system dysfunctions play a pivotal role in the pathogenesis of COVID-19. Furthermore, the quest of finding a potential pharmaceutical therapy for preventing and treating COVID-19 is still ongoing. Silymarin, a mixture of flavonolignans extracted from the milk thistle, has exhibited numerous therapeutic benefits. We reviewed the beneficial effects of silymarin on oxidative stress, inflammation, and the immune system, as primary factors involved in the pathogenesis of COVID-19. We searched PubMed/Medline, Web of Science, Scopus, and Science Direct databases up to April 2022 using the relevant keywords. In summary, the current review indicates that silymarin might exert therapeutic effects against COVID-19 by improving the antioxidant system, attenuating inflammatory response and respiratory distress, and enhancing immune system function. Silymarin can also bind to target proteins of SARS-CoV-2, including main protease, spike glycoprotein, and RNA-dependent RNA-polymerase, leading to the inhibition of viral replication. Although multiple lines of evidence suggest the possible promising impacts of silymarin in COVID-19, further clinical trials are encouraged.
Abbreviations: ACE2, angiotensin converting enzyme; AMPK, AMP-activated protein kinase; APC, antigen-presenting cells; COVID-19, coronavirus disease 2019; ERK, extracellular signal-regulated kinase; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IFNγ, interferon γ; IL, interleukin; JAK, c‐Jun N‐terminal kinase; MAPK, mitogen-activated protein kinase; MERS-CoV, Middle East respiratory syndrome coronavirus; NF-κB, nuclear factor kappa B; NLR, neutrophil-lymphocyte-ratio; NO, nitric oxide; Nrf2, nuclear factor erythroid 2–related factor 2; ROS, reactive oxygen species; SARS, severe acute respiratory syndrome; SARS-CoV 2, severe acute respiratory syndrome coronavirus; STAT, signal transducer and activation of transcription; TGF-β, transforming growth factor beta; Th1, T helper 1; TLR-4, toll like receptor 4; WHO, World Health Organization
Keywords: COVID-19, SARS-COV-2, Silibinin, Inflammation, Oxidative stress, Immune system, Review
Graphical Abstract
1. Introduction
Late in 2019, an unknown coronavirus, later entitled severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was detected in patients suffering from pneumonia of unknown etiology in Wuhan, China [1], [2], [3]. The unrevealed nature of SARS-CoV-2 and its rapid respiratory transmission brought about a vigorous pandemic in the world [3]. As of April 2022, the Coronavirus disease 2019 (COVID-19) has spread rapidly throughout the world and infected more than 490 million individuals, leading to more than six million deaths [4]. According to previous studies, patients with COVID-19 may exhibit a broad, unspecific spectrum of signs and symptoms, including fever, sore throat, nausea, vomiting, myalgia, and dizziness [5], [6]. Although most COVID-19 patients recover within weeks, about five percent of patients may experience a severe lethal disease leading to multi-organ damage, ARDS, and death [7], [8]. In addition, elder individuals or patients with various co-morbidities are susceptible to severe COVID-19 [9]. Despite vaccine development, people worldwide are dealing with new variants of SARS-CoV-2 such as delta, lambda, and omicron [10]. In this regard, the quest to find a potential therapeutic drug is still ongoing.
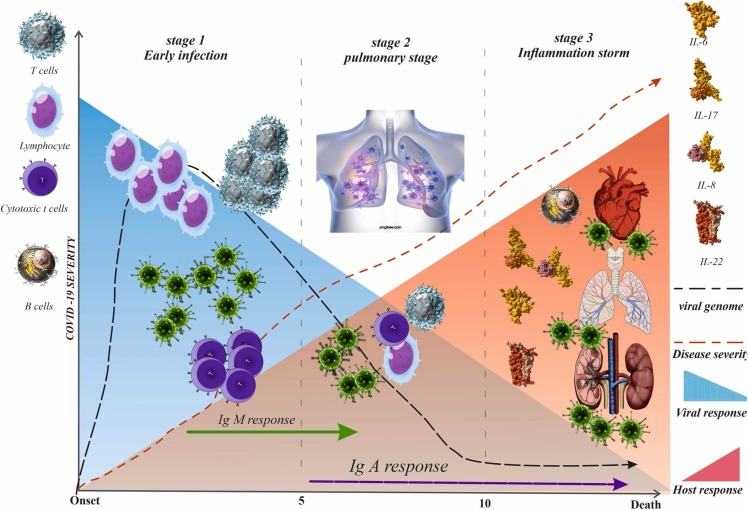
Though the exact pathophysiology of COVID-19 is not fully understood so far, studies have shown that the clinical course of COVID-19 can be classified into three stages: initial viral infection, pulmonary stage, and hyper-inflammation stage ( Fig. 1) [11], [12]. It is assumed that the cytokine storm and hypercoagulation of the hyper-inflammation phase contribute to disease severity and mortality [13]. In this regard, critically ill patients with COVID-19 have displayed higher levels of neutrophils, pro-inflammatory cytokines, and ROS [14]. The latter, in turn, is thought to be associated with endothelial cell dysfunction, impaired immune response, and platelet aggregation, which all exacerbate the severity of COVID-19 patients [15]. In the present emergent situation, drug repositioning from approved and well-known complementary medicine for developing possible therapeutic and prophylactic agents may be cost-effective [16]. An ideal beneficial approach to manage COVID-19 would contain a drug capable of directly targeting the virus lifecycle while preventing hypercoagulability and cytokine storm [17]. Flavonoid and polyphenol compounds such as luteolin, quercetin, genistein, and silymarin are known as natural ligands of peroxisome proliferator-activated receptor-γ, which decreases cytokine levels and suppresses the inflammatory pathways. Consequently, they might play a protective role against COVID-19-associated complications [18]. In this regard, polyphenol and flavonoid compounds' anti-inflammatory and antioxidant activity have been confirmed strongly [19], [20]. Silymarin is a complex polyphenolic compound derived from the seed of the milk thistle plant, and it is known for its anti-inflammatory, antiviral, anti-oxidative, and immunomodulatory effects [21], [22], [23]. Silymarin was reported to inhibit the hepatitis C virus in vivo and in vitro by inhibiting hepatitis C virus entry, viral protein expression, RNA synthesis, and virus replication. It also inhibits the virus's cell-to-cell spread [24], [25]. Silibinin, a combination of two stereoisomers, silibinin B and silibinin A, in equimolar ratio, is the main component of this complex extract and is the most active constituent of silymarin [26], [27]. In particular, silibinin decreases viral load in patients with chronic hepatitis C [28]. Silibinin can affect viral RNA-dependent RNA polymerase action, preventing hepatitis C virus replication [29]. Silibinin has been reported for its anti-inflammatory and anti-oxidative activity and protective effects against endothelial dysfunction in both in vitro [30] and in vivo [22] studies. Nieuwenhuizen et al. concluded that silymarin has a protective effect against lung injury due to its ability to decrease the production of nitric oxide, the infiltration of inflammatory cells, the activity of myeloperoxidase, and its ability to decrease the protein levels of pro-inflammatory mediators, catalase, superoxide dismutase, and GSH peroxidase [31]. Moreover, silymarin has recently been considered a potent inhibitor for angiotensin converting enzyme-2 (ACE-2), preventing its host-cell entry [32]. Therefore, therapeutic agents with antiviral, anti-inflammatory, and immunomodulatory activities may benefit the treatment of COVID-19. We, therefore, aimed to investigate the possible favorable impacts of silymarin in the treatment of COVID-19 by conducting a comprehensive review of published research.
Fig. 1.
The phases of COVID-19. The COVID-19 can be divided into 3 stadiums: the early infection, the pulmonary and the hyperinflammation stages. In the early infection, the viral load (purple line in the blue zone) starts to increase and at some points, it begins to activate the host immune response (red zone). While the disease progresses into a more severe state, the proinflammatory cytokines build up and start to form antibody against the virus. When the disease is not promptly treated, COVID-19 may fall into the hyperinflammation stage, multiorgan failure and death.
2. Methods
2.1. Data sources and search strategy
In the present study, we searched the electronic databases PubMed/Medline (28 studies), Web of Science (53 studies), Scopus(33 studies), and Science Direct (37 studies), using the following keywords: “milk thistle” [Title/Abstract] or “Silybum marianum L. Gaertn [Title/Abstract] ” or “silymarin” [MESH], or “ silibinin” [MESH], or “C25H22O10” [Title/Abstract] or “silymarin isomers” [Title/Abstract] and “viral diseases” [Title/Abstract] or “virus-related diseases” [Title/Abstract] or “inflammation” [Title/Abstract] or “immune system [Title/Abstract]” or “oxidative stress” [Title/Abstract] or “COVID-19” [MESH] or “SARS-CoV-2” [MESH] or “coronaviruses [Title/Abstract] ”. Relevant peer-reviewed studies published in English until April 2022 were included in this study. Articles with inadequate data were excluded from the review. The reference of all the obtained articles was also checked to identify other relevant publications. The retrieved studies were screened using the EndNote software (Thomson Reuters, USA), and duplicate reports were removed. The remaining studies were evaluated to identify related articles within the scope of this study. Finally, full texts of the eligible studies were assessed.
2.2. Inclusion criteria
The inclusion criteria were: [1] All in-vitro, animal, and clinical trials and [2] Silibinin and Silymarin supplements should be used alone, and [3] Articles published in English.
2.3. Exclusion criteria
The exclusion criteria were [1] studies investigating the effect of silibinin and silymarin on other diseases and [2] articles reported in other languages.
2.4. Data extraction
Two authors (A.K. and M.V) assessed the full text of selected articles and screened them for data extraction. Any study's extracted data consisted of the authors' name, the issue, and the main conclusion of the study. A third author (M.V) evaluated the accuracy and quality of the extracted data.
3. Results
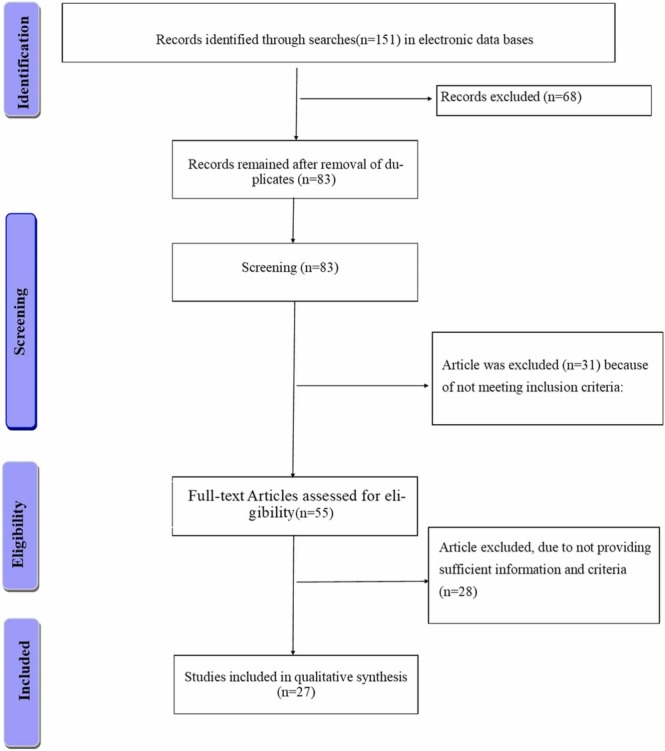
One hundred fifty-one articles were identified in the primary search. Of these, 69 articles were removed in duplicate checking, and 55 were not within the scope of the study. Finally, 27 articles were selected for the final assessment and review. The flow chart of selecting obtained articles is depicted in Fig. 2 .
Fig. 2.
Flow diagram of the literature search and study selection process.
3.1. The Pathogenesis of COVID-19
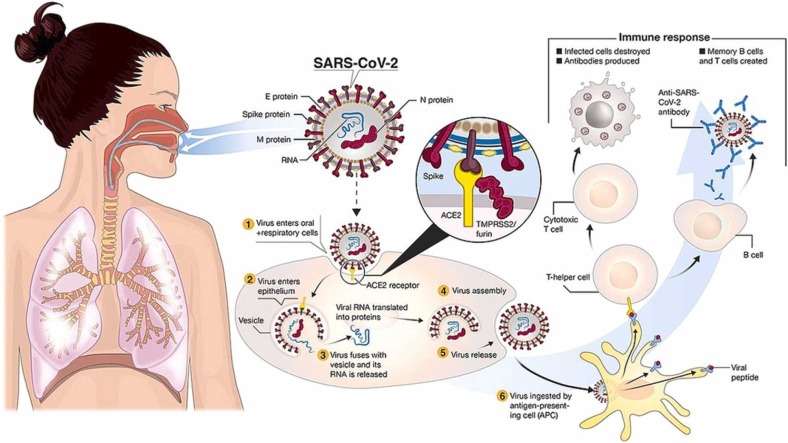
The pathogenesis of COVID-19 has not been fully identified yet [33]. However, there are several hypotheses regarding the pathogenesis of COVID-19 [33]. In this regard, it is assumed that SARS-CoV-2 bind to angiotensin-converting enzyme 2 (ACE2) receptors of alveolar epithelial cells through the surface glycoproteins [34]. After binding, the spike proteins of SARS-COV-2 are fragmented via acid-dependent proteolysis through TMPRRS2, cathepsin, or furin protease, and viruses enter the cytoplasm via endocytosis [35], [36]. Then, the genomes of the viruses release and translated into target proteins in the cytoplasm. In addition, it should be noted that the attachment of SARS-Cov-2 to ACE2 receptors elevate the expression of ACE2 receptors at the membrane of alveolar cells, facilitating the further entrance of the virus and leading to alveolar damage [37], [38].
Studies have shown that the primary mechanism of COVID-19 is the onset of inflammatory cytokine storms [39]. When immune cells are exposed to viral infection, many pro-inflammatory factors such as interferons (IFN-α, IFN-γ), interleukins (IL-33, IL-6, IL-1β, IL-12, IL-18), tumor necrosis factor‐α (TNF-α) and transforming growth factor-beta (TGF-β), and chemokine ligands (CXCL9, CCL3, CXCL8, CCL5, CCL2, CXCL10) are released into the infected tissue [40]. Qin et al. [41] observed a decrease in lymphocytes, an increase in leukocytes and neutrophil-lymphocyte-ratio (NLR), and eosinophils, basophils, and lower percentages of monocytes, in patients with severe COVID-19. In addition, T cells, including helper T cells and regulatory T cells, are inhibited in severe cases of COVID-19 patients [41].
In addition, critically ill patients with COVID-19 are shown to have elevated amounts of oxidative stress (OS) [42]. Oxidative stress, defined as the imbalance of oxidative agents and antioxidant defense, initially aims to eradicate the invading pathogen [43]. However, if exceeded may impair the immune function itself. The excessive oxidative stress in COVID-19 patients is assumed to be due to the inhibition of NRF2- mediated pathways and NF- κB signaling activation [44].
A considerable feature of MERS-CoV and SARS-CoV is the production of double-layered membrane vesicles (DMVs) that the processes of transcription occur within them [45]. Additionally, autopsy and histopathological investigation of the kidney tissue in patients with COVID-19 indicated that SARS-CoV-2 might exist inside the DMVs of the host cells [46]. Therefore, they cannot be identified by the host immune system [47]. Furthermore, one of the dominant pathogenic properties of MERS is its ability to inhibit the host bronchial interferon synthesis [48]. Likewise, experimental studies showed that SARS-CoV-2 poorly stimulates the IFN-I response, which is crucial in the combat against viruses [49].
Moreover, another suggested mechanism for the pathogenesis of COVID-19 is the increased neutrophil extracellular traps (NETs), which are released by neutrophils in response to invading pathogens [50]. In addition, neutrophils may also be responsible for the thrombosis and multi-organ damage of COVID-19 through neutrophil reverse transendothelial migration (rTEM) [51]. Studies have shown that a pre-existing upper respiratory tract viral infection may susceptible the patients to severe bacterial pneumonia and exacerbate the inflammatory response [52]. In this regard, the robust immune response to the SARS-CoV-2 triggers the release of cytokines and a chemokine cascade that results in a hyper-inflammatory state and accumulation of fluid in the lungs [53], [54]. Overall, it seems that impaired immune response, the escape mechanism of SARS-Cov-2, and increased inflammatory cytokines and cytokine storm contributes to the pathogenesis of COVID-19, leading to possible ARDS and multi-organ damage [55]. In the current review, we proposed to investigate the effects of silymarin, a flavonoid derived from the medicinal plant Silybum marianum (L.) Gaertn, on some of the important factors involved in the pathogenesis of COVID-19 including cell-mediated immunity, inflammation, viral attachment to the cell surface, cell membrane disruption, inhibition of interferon synthesis, and accumulation of fluid in the lungs.
3.2. Silymarin
Silybum marianum L. Gaertn, or milk thistle, as one of the common herbal remedies, contains various chemical compounds with healing properties. The most common prevalent compound of milk thistle is silymarin [56]. Silymarin, a vital complex extracted from milk thistle, consists of four isomers of flavonolignans, including silybin, isosilybin, silydianin, and silychristin. The general empirical formula of silymarin is C25H22O10. Silybin is one of the most prevalent and active isomers of silymarin, with a concentration of about 60–70 % [57]. Silymarin has been indicated to possess various pharmacological functions such as hepatoprotective, antioxidant, anti-inflammatory, cardioprotective, and antiviral activities [58]. Numerous studies have examined the effects of silymarin on liver disease [59]. Additionally, some studies demonstrated the therapeutic properties of milk thistle on viral diseases, including hepatitis C (HCV) and influenza [60], [61]. A brief review of studies evaluating the possible antiviral effects of silymarin is presented in Table 1.
Table 1.
Summary of studies evaluating antiviral effect of silymarin.
| Articles | Type of study | Reference | Samples | Study design | Main results |
|---|---|---|---|---|---|
| Viral-related articles | In vitro | Polyak et al. [1] | HCV-infected Huh7 and Huh7.5.1 cells | Administration of silymarin: 10, 20, 40, 100 µg/ml | Inhibition of expression of TNFα and NF-kB; prevention of infection by JFH-1 virus; displaying prophylactic and therapeutic effects against HCV infection |
| In vitro | Wagoner et al. [2] | HCV-infected Huh7 and HepG2 cells | Administration of silymarin: 0–120 µM | Inhibition of virus entry, RNA and protein expression, and infectious virus production; prevention of cell-to-cell spread of virus; inhibition of JFH-1 genotype 2a NS5B-dependent RNA polymerase activity | |
| In vitro | Song et al. [3] | Influenza A virus-infected MDCK cell | Administration of silymarin: 0–100 µg/ml | Exhibition of anti-influenza A virus activity of 98 %; inhibition of viral mRNA synthesis | |
| In vitro | McClure et al. [4] | HIV-infected TZM-bl cells | Administration of silibinin: 0–324 µM | Inhibition of HIV-1 replication; reduction of actively proliferating CD19+, CD4+, and CD8+ cells; attenuation of cellular functions involved in T-cell activation, proliferation, and HIV-1 infection | |
| In vitro In vivo |
Dai et al. [5] | BALB/c mice, MDCK, A549, and Vero cells | An assay based on the inhibition of the formation of the Atg12-Atg5/Atg16 heterotrimer | Reduction of influenza A virus replication; reduction of mortality in infected mice | |
| In vitro | Lani et al. [6] | CHIKV-infected Vero cells | Administration of silymarin: 0–200 µg/ml | Antiviral activity against CHIKV; reduction of CHIKV replication efficiency; down-regulating production of viral proteins involved in replication | |
| In vitro | Hanafy, El-Kemary [7] | Mice | Administration of silymarin: 96 ± 0.3 μg/ml | his pathological profile was significantly remodulated by encapsulated silymarin. In mean well, IL-6 and CRP were significantly reduced in oleic acid model as well after treatment. Additionally, encapsulated silymarin exhibited antiviral activity against COVID19 by using plague reduction assay |
|
| In vitro | Morishima, Shuhart, Wang, Paschal, Apodaca, Liu, Sloan, Graf, Oberlies, Lee [8] | Freshly isolated peripheral blood mononuclear cells (PBMC) and T cells from HCV-infected |
silymarin (MK001), 5 and 40 µM /ml | silymarin (MK001), dose dependently inhibited the proliferation and secretion of TNF-α, IFN-gamma, and IL-2 by PBMC stimulated with anti-CD3. In addition, MK001 inhibited proliferation by CD4+ T cells to HCV, Candida, and tetanus protein antigens and by HLA-A2/HCV 1406–1415-specific CD8 T cells to allogeneic stimulation. MK001 inhibited T-cell TNF-α and IFN-gamma cytokine secretion to tetanus and Candida protein antigens. Finally, MK001 inhibited nuclear factor-kB transcriptional activation after T-cell receptor-mediated stimulation of Jurkat T cells, consistent with its ability to inhibit Jurkat T-cell proliferation and secretion of IL-2. |
|
| In vitro | Camini, da Silva, da Silva Caetano, Almeida, Ferraz, Vitoreti, de Mello Silva, de Queiroz Silva, de Magalhães, de Brito Magalhães [9] | MAYV-infected HepG2 | silymarin (MK001), 3.125 and 100 µM /ml | silymarin could reduce MAYV-induced oxidative cell damage. Briefly, silymarin exhibited potent antiviral activity against MAYV and reduced MAYV-induced ROS formation and levels of malondialdehyde (MDA) and carbonyl protein, which are biomarkers of oxidative stress. | |
| In vitro | Lovelace, Maurice, Miller, Slichter, Harrington, Magaret, Prlic, De Rosa, Polyak [10] | Monocyte and MAIT cell | Silymarin, 80 μM | silymarin treatment suppressed the expression of T cell activation and exhaustion markers on CD4+ and CD8+ T cells from chronically-infected, HIV-positive subjects. silymarin also showed a trend towards modifying CD4+ T cell memory subsets from HIV+ subjects. In the HIV-negative setting, silymarin treatment showed trends towards suppressing pro-inflammatory cytokines from non-activated and pathogen-associated molecular pattern (PAMP)-activated primary human monocytes, and non-activated and cytokine- and T cell receptor (TCR)-activated mucosal-associated invariant T (MAIT) cells. | |
| In vitro | Meroni, Barcellini, Borghi, Vismara, Ferraro, Ciani, Zanussi [11] | Lymphocyte Blastogenesis | Silybin,0.5, 10, and 25 μM | silybin on activating human T lymphocytes and observed that silybin significantly reduce the proliferative reaction to the monoclonal anti-CD3 antibody in a dose-dependent manner | |
| In vitro | Hawke, Schrieber, Soule, Wen, Smith, Reddy, Wahed, Belle, Afdhal, Navarro, Berman, Liu, Doo, Fried [12] | 32 patients with chronic HCV infection | Silymarin, 140, 280, 560, or 700 mg | clinically meaningful reductions from baseline serum transaminases or HCV RNA titer were observed. | |
| Case report | Payer et al., 201013 | A case of an HIV-HCV coinfected patient | Administration of silibinin: 20 mg/kg/day for 14 days intravenously | Inhibition of HIV replication; a decrease in HCV RNA and HIV RNA | |
| Clinical trial | Yakoot et al. [14] | 66 patients with chronic HCV infection | Administration of silymarin: 140 mg 3 times daily for 6 months | No virological response in the 96.6 % of silymarin treated group | |
| Clinical trial | Biermer et al. [15] | 20 patients with chronic HCV | Administration of silibinin: 1400 mg daily, infusion on 2 consecutive days | Complete viral suppression in 13 of 20 patients; remaining HCV RNA negative during the subsequent follow up period | |
| Clinical trial | Adeyemo et al. [16] | 32 patients with chronic HCV | Administration of silymarin: 420 mg 3 times daily and 700 mg 3 times daily for 20 weeks | No alteration in serum ALT and HCV RNA titers; suppression of C. albicans-induced T-cell IFNγ and phytohemagglutinin-induced T-cell proliferation; modest non-specific immunomodulatory effects in vivo by silymarin administration | |
| COVID-19 related article | |||||
| Molecular docking analysis | Latha et al. [17] | Phytochemicals from the medicinal plants | Docking analysis | Better binding affinity to the target proteins of SARS-COV-2 than the synthetic repurposed drugs for treatment of COVID-19 | |
| Molecular docking analysis | Saraswat, Singh, Patel [18] | Phytochemicals from the medicinal plants | Docking analysis | The docking results showed successful binding to the active site or near a crucial site. The present computational approach was found helpful to predict the best possible inhibitor of protease and may result in an effective therapeutic agent against COVID-19. | |
| In vitro | Speciale, Muscarà, Molonia, Cimino, Saija, Giofrè [19] | HUVECs | 5,10 and 25 µg/ml | silibinin reduced TNF-α-induced gene expression of the proinflammatory genes IL-6 and MCP-1, as well as of PAI-1, a critical factor in coagulopathy and thrombosis, and of ET-1, a peptide involved in hemostatic vasoconstriction. Then, due to endothelium anti-inflammatory and anticoagulant properties of silibinin and its capability to interact with SARS-CoV-2 main target proteins demonstrated herein, silibinin could be a strong candidate for COVID-19 management from a multitarget perspective. | |
| molecular docking experiments | Phytochemicals from the medicinal plants | Docking analysis | |||
| molecular docking experiments | Patel, Goswami, Sivakumar, Pandya [20] | Phytochemicals from the medicinal plants | molecular docking and molecular dynamics (MD) simulations |
silymarin lead to possessing the ability to interact and mask the amino acids of RBD, making them unavailable to form associations with ACE2. Such a molecule is termed as ‘fusion inhibitor’. We hypothesized to identify fusion inhibitors from the NPACT library of anticancer phytochemicals. | |
| molecular docking experiments | Patel, Kumar, Pandya, Rawal [21] | CoV-2 hemagglutinin-acetylesterase (HE) glycoprotein as |
Docking analysis | Silymarin, as potential hemagglutinin-acetylesterase (HE) glycoprotein inhibitors with better binding energy. | |
| In vitro | Aguilar‑Lemarroy, López‑Uribe, Sánchez‑Corona, Jave‑suárez [22] | HaCaT, DOK, A549, H1299 and Lenti-X 293 T cells | transcrip¬tion quantitative PCR Use of viral particles containing SARS CoV 2 ORF3a and bioinformatics |
silymarin significantly decrease the level of ACE2 expression a In addition, silymarin treatment markedly decreased IL-6, TNF-α RPL18, RPL32 interleukin‑18 mRNA levels. The combination of phytonutrients in silymarin may help to boost the immune system and could reduce the effects of COVID‑19. | |
| molecular docking experiments | Gorla, Rao, Kulandaivelu, Alavala, Panda [23] | Phytochemicals from the medicinal plants | Molecular Docking Studies (482.44 g/mol) | silymarin bind significantly at the active sites of RBD-S and PD-ACE-2 with a MolDock score. | |
| molecular docking experiments | Srivastava, Tripathi, Unni, Hussain, Haque, Dasgupta, Singh, Mishra [24] | Phytochemicals from the medicinal plants | Molecular Docking Studies | -Silybin, with their possible potential effectiveness in the treatment of COVID-19, reflect future possibilities in viral protease inhibition by the use of flavonoids -Silybin B demonstrated better binding and ADME properties compared with the currently endeavored drugs like Hydroxychloroquine and Lopinavir. |
|
| molecular docking approach | Kumar, Kashyap, Chowdhury, Kumar, Panwar, Kumar [25] | Phytochemicals from the medicinal plants | Docking analysis | The present study demonstrated the binding potential of silymarin with Nsp15 and is capable of inhibiting viral replication, | |
| In vitro | Loutfy, Abdel-Salam, Moatasim, Gomaa, Fattah, Emam, Ali, ElShehaby, Ragab, El-Din [26] | Vero and Vero E6 cell lines | 6.25, 12.5, 25, 50, 100, and 200 μg/m | Silymarin against SARS-CoV-2 was through interference with viral attachment by blocking ACE2 receptor. | |
| Clinical trials | Aryan, Farahani, Chamanara, Elyasi, Jaafari, Haddad, Sani, Ardalan, Mosaed [27] | silymarin | 70 mg | The present study demonstrated there were not significant differences between the two groups in terms of symptoms resolution time, laboratory parameters (Serum creatinine level, C-reactive protein, Lymphocyte count, Atrial O2 saturation, Length of need for supplement of O2 AST), and hospitalization duration. However, the alanine aminotransferase level decreased significantly in the treatment group, compared to the placebo group. |
ALT, alanine transaminase; CHIKV, chikungunya virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IFN-α, interferon-alpha; MAYV, mayaro virus; MDCK, madin-darby canine kidney; NF-kB, nuclear factor kappa B; TNF-α, tumor necrosis factor-alpha; Vero cells, african green monkey kidney cells.
3.3. Cell-mediated immunity effects of silymarin
As mentioned above, cell-mediated immunity is one of the pathways contributing to the pathogenesis of COVID-19. Previous studies investigating the impacts of silymarin on secondary cell-mediated immunity in liver damage condition has revealed that silymarin can decrease whole circulating T lymphocytes, reduce delayed hypersensitivity, and increase the cytotoxicity of killer T lymphocytes sensitization [62]. Likewise, experimental studies demonstrated that silymarin might increase the levels of IL-12 in the draining lymph nodes by appending the number of antigen-presenting cells (APC). Furthermore, the IL-12 stimulates the production of IFN-γ, which improves T-cells function, exclusively T helper 1 (Th1) [63], [64], [65]. Moreover, it has been shown that silymarin can inhibit the immune suppression of UVB radiated mice by decreasing the elevated levels of IL-10 in both the skin and draining lymph nodes [66], [67]. Meroni et al. [68] examined the effect of dosages of silybin (0.5, 10, and 25 0.5, 10, and 25 μM) on activating human T lymphocytes. They observed that silybin significantly reduces the proliferative reaction to the monoclonal anti-CD3 antibody in a dose-dependent manner. In addition, they reported an increase in proliferation of either alloantigen or mitogen-stimulated lymphocytes in a dose-dependent manner alongside an enhancement of IFN-γ, IL-4, and IL-10 secretion [68], [69].
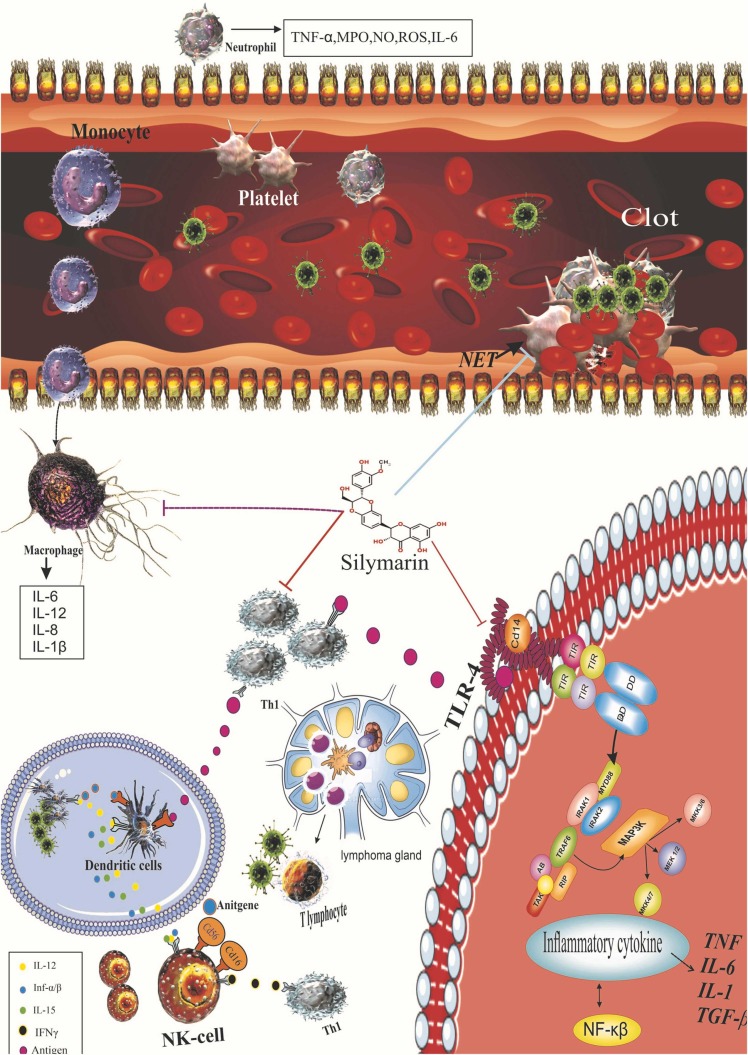
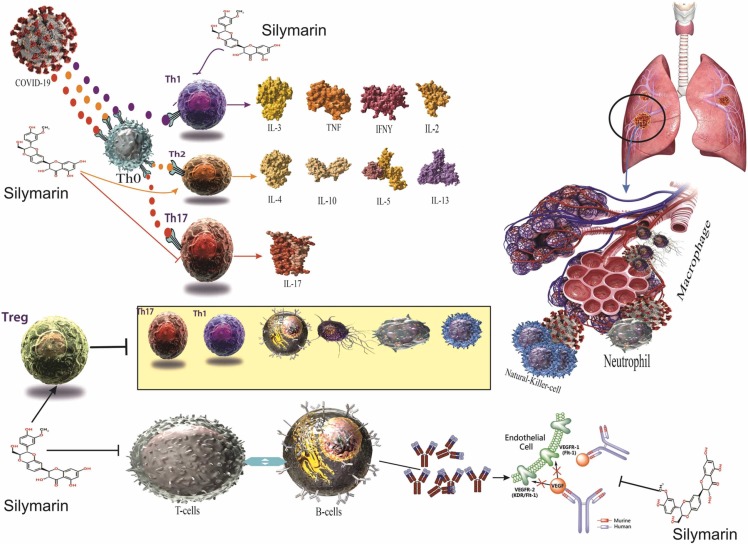
Furthermore, silymarin improved macrophage activation, nitric oxide (NO) and macrophage phagocytosis, lysozyme contents, and immune indices of spleen and thymus in immunosuppressive mice, leading to the amelioration of non-specific immune functions and also protection against infectious agents [70], [71]. The mechanisms of actions of silymarin on the immune system are presented in Fig. 3. The role of cell-mediated immunity against SARS-CoV-2 infection is still unknown [72]. Nevertheless, immunity cells, including B cells, CD8+ T cells, CD4+ T cells, total lymphocytes, and natural killer cells, exhibited a significant correlation with inflammatory status in patients with COVID-19. On this subject, post-treatment reduced B cells, and CD8 + T cells and elevated CD4 + /CD8 + ratio were considered unresponsive to treatment in the COVID-19 patients [73]. Besides, patients with severe SARS-CoV-2 infection exhibited a considerable reduction in the expression of IFN-γ, which is stimulated through CD4 + T cells [74]. Furthermore, Lovelace et al. [75] observed that silymarin considerably decreases the pro-inflammatory cytokines and modulates the immune response by inhibiting the expression of T cell stimulation in HIV patients.
Fig. 3.
Anti-inflammatory and anti-oxidative stress effects of silymarin. Sallymarin increases the transcriptional activity of Nrf2. Regulation of the expression of antioxidant genes is critical for controlling oxidative stress and maintaining physiological homeostasis. Of the various regulatory pathways, the Keap1-Cul3-Rbx1, Antioxidants axis is the most important regulator of Nrf2 activity. Sallymarin also reduces the expression of the TLR-4 pathway, which leads to a decrease in NF-KB activity and the production of inflammatory mediators. Silymarin also modulates the immune response, leading to the production of NETS by noutrophil and reducing the overproduction of inflammatory and oxidative factors by immune responses. Anti-inflammatory effects of silymarin. TLR, Toll-like receptor; NF-κB, nuclear factor kappa B; IkB, inhibitor of kappa B; PUSFA, polyunsaturated fatty acids; TNF-α, tumor necrosis factor-alpha; IFNs, interferons; IL, interleukin.
3.4. Anti‐inflammatory and antioxidant activity of silymarin
Oxidative stress and inflammation play critical roles in the pathogenesis of infectious diseases, including COVID-19. A current study by Chiappetta et al. [76] found that higher basal levels of inflammatory cytokines, including CRP (C-reactive protein) and IL-6, contribute to the impaired immune response. This, in turn, results in prolonged pro-inflammatory responses and impaired control of viral replication in obese patients with COVID-19. In addition, a recent meta-analysis on the role of immune-inflammatory factors in COVID-19 cases indicated that critically ill patients tend to exhibit elevated inflammatory markers, including IL-6, CRP, and ESR (erythrocyte sedimentation rate) compared to non-severe patients [77]. Another recent systematic review on the association of oxidative stress and SARS-CoV-2 infection suggested that oxidative stress is the main factor determining the severity of COVID-19 [78]. In this regard, neutrophils, macrophages, and immune-inflammatory cells, responsible for producing the majority of oxidants in the lung tissue, are increased in patients with COVID-19 [79], [80]. However, further studies are encouraged to identify the impact of chronic inflammation and oxidative stress in the pathogenesis of SARS-CoV-2 infection [76].
Studies on the anti-inflammatory effects of silymarin have revealed that it can down-regulate the release of cytokines (TNF‐α and IL-1) and adhesion molecules (E-selectin) by inhibition of nuclear factor-kappa B (NF‐κB) signaling, NO, and 5–lipoxygenase cascade [59]. Silymarin inhibits mitogen‐activated protein kinase (MAPK) activation by suppressing TNF‐α, and c‐Jun N‐terminal kinase (JNK) action cascades TNF‐α‐induced cytotoxicity and caspase activation. Furthermore, silymarin suppresses the binding of NF‐κB on DNA through the attenuation of the IkB phosphorylation, which leads to the translocation of NF‐κB to the nucleus without binding to the DNA [81], [82], [83].
In a study by Li et al. [84], they indicated that silymarin decrease smoke-induced airway inflammation by suppressing the activity of extracellular signal-regulated kinase/p38 mitogen-activated protein kinase (ERK/p38 MAPK) pathway and autophagy in human bronchial epithelial cells. Furthermore, silymarin inhibits apoptosis and gene expression of toll-like receptor 8 (TLR8) in the Ramos cancer cell line [85]. Additionally, silymarin decreases the conversion of polyunsaturated fatty acid to leukotrienes via inhibiting the lipoxygenase enzyme [86]. Mechanisms of action of silymarin on inflammation are presented in Fig. 4.
Fig. 4.
Immunomodulatory effects of silymarin. silymarin has the ability to decrease the expression of Th1 cytokines (TNF‐α, IL‐12, IL‐1, and IFN‐γ) in CD4 + T cells through suppressing IL‐12 production in macrophages and arise the Th2 cytokines expression (IL‐10 and IL‐4). Also, silymarin blocks expansion and differentiation of Th17 cells by inhibiting the expression of IL‐17, IL‐6, IL‐21, and related orphan receptor gamma t (RORγt) signaling and signal transducers and activators of transcription 3 (STAT3) phosphorylation.
Oxidative stress usually occurs after an inflammatory condition. In this regard, the destruction of cells after exposure to infectious agents leads to extensive tissue damage. Many studies confirmed the antioxidant effects of silymarin on liver diseases. The mechanisms of actions of silymarin on oxidative stress include suppression of reactive oxygen species (ROS)‐producing enzymes, ability to scavenge free radicals, chelation potency for both copper and iron, and increased synthesis of protective molecules such as sirtuins, thioredoxin, and heat shock proteins that protect against stressful stimuli [87]. Also, silymarin activates superoxide dismutase (SOD) and non-enzymatic pathways through nuclear-related factor 2 (Nrf2) activation. In this regard, it has been reported that it significantly increases the expression of SOD in patients with nonalcoholic steatohepatitis [88]. Moreover, studies have shown that the administration of silymarin decreases oxidative stress in patients with β‐thalassemia [89], [90]. In this regard, in an in vitro study by Hanafy and El-Kemary [22], they demonstrated that silymarin improves the histopathology profile of lung tissue in COVID-19 and reduces the release of nitric oxide, pro-inflammatory cytokine, superoxide dismutase, catalase, and GSH peroxidase.
3.5. Antiviral effects of silymarin and its possible mechanisms of action against COVID-19
Silymarin has been suggested to have multiple biological functions against various viruses [91], [92]. The antiviral effects of silymarin from in vitro, in vivo, clinical trial, case-control, and bioinformatic studies are reviewed in this section [91]. These studies assessed the Janus kinase (JAK)-signal transducer and activation of transcription (STAT) signaling pathway, polymerase activity, protein synthesis, entrance, and transmission of viruses [93]. Also, the expression of interferons, anti-inflammatory and antioxidant effects of silymarin were evaluated. IFN-γ is one of the cytokines released from infected cells and affects adjacent cells, making them vulnerable to virus invasion [94]. Pathological studies revealed that SARS-CoV-2 inhibits interferons synthesis [95], [96]. Silymarin regulates the levels of IFN-γ through the stimulation of T-cells, particularly Th1 type cells [97], [98]. Antiviral effects of silymarin have been evaluated in viruses including HCV, influenza A virus, mayaro virus (MAYV), chikungunya virus (CHIKV), and human immunodeficiency virus (HIV). In all mentioned viruses, silymarin has exhibited antiviral activity by preventing the virus from entering the host cells, genomic content replication, and the expression of viral protein genes [99]. In a study, Lani et al. [100] assessed the effects of silymarin components on CHIKV genome replication. They demonstrated an antiviral activity of silymarin against CHIKV by attenuation of genome replication (93.4 %) and protein expression (more than 99 %). An in vitro study regarding the antiviral effect of silymarin on MAYV demonstrated that silymarin inhibits MAYV replication and decreases MAYV-induced oxidative stress [101].
Similar to influenza virus, SARS-CoV-2 is a single strand RNA virus. Recent studies suggested that some drugs which are administered for the treatment of influenza may also be effective in treating COVID-19 symptoms [102]. According to a study, administration of silymarin at the dosages of 100 µg exhibited 98 % antiviral activity against the influenza A virus by inhibiting viral mRNA synthesis [60]. Furthermore, in another study, Dai et al. [103] reported that silybin reduces the replication of influenza A. In addition, in another study on the antiviral effects of silibinin, the authors demonstrated that silibinin inhibits the replication of HIV-1 in TZM-bl cells. Additionally, cellular functions involved in proliferation, T-cell activation, and HIV-1 infection are attenuated by the administration of the intravenous formulation of silibinin [104].
Several in vivo and in vitro studies examining the effects of silymarin on HCV revealed that silymarin attenuates the replication and survival of HCV by preventing viral entry to the cells and activating the JAK-STAT antiviral signaling pathway [92], [93]. Also, silymarin inhibits HCV's RNA-dependent RNA polymerase activity and decreases its function [105]. Torres et al. [106] indicated that silymarin administration decreases the load of the hepatitis C virus. There are limited human studies regarding the impacts of silymarin on hepatitis C. The results of these studies suggested that silymarin does not have any significant effect on the replication rate of HCV. In contrast, it improves the antioxidant levels and immune system function in infected individuals [107], [108], [109]. In a study, administration of silymarin (140 mg 3 times a day for six months) in 66 patients with chronic HCV infection exhibited no virological response in the 96.6 % of silymarin treated group [107]. In addition, a recent study demonstrated that silymarin might be a potent inhibitor of the hemagglutinin esterase (HE) glycoprotein receptor [110]. The HE acts as a receptor-destroying enzyme in the binding of SARS-CoV-2 to the ACE receptors. Besides, studies indicated that silymarin might inhibit the host-cell entrance of SARS-Cov-2 by inhibiting ACE-2 receptors [30]. Zhang et al. [111] suggested that a timely anti-inflammation therapy, including glucocorticoids, JAK inhibitors, and IL-6 antagonists, in COVID-19 patients may have the most beneficial effects. Due to silymarin's anti-inflammatory, antioxidant, antiviral, and immunomodulatory impacts, silymarin may be a promising treatment option against COVID-19 [100], [112]. A bioinformatics study about the possible mechanisms of actions of silymarin on COVID-19 demonstrated that silymarin had a high binding affinity to target proteins of SARS-CoV-2, including spike glycoprotein, main protease, and RNA-dependent RNA-polymerase compared to current drugs administering in the treatment of COVID-19. The docking results exhibit that silymarin can bind to SARS-CoV-2 main protease, spike glycoprotein, and RNA-dependent RNA-polymerase with docking energy of −11.928 kcal/mol, −10.572 kcal/mol, and −11.499 kcal/mol, respectively [113]. Recent studies have found that substances with RNA-dependent RNA polymerase inhibitory activity can be used as a possible beneficial drug in the treatment of COVID-19 [114]. Speciale and colleagues [28] conducted an in silico and in vitro study to investigate the impact of Silibinin administration on COVID-19. They demonstrated that the silibinin could interact with spike proteins of SARS-CoV-2 and inhibit the entrance of the virus. In addition, they observed that silibinin considerably decreases the expression of TNF-α and inflammatory cytokines such as genes MCP-1, IL-6, and PAI-1 [28]. The latter is an indicator of endothelial dysfunction, which contributes to the thrombotic events of COVID-19 [115]. However, one of the limitations of such studies was the lack of clinical trials or very few human studies which are encouraged to investigate in detail the mechanism of action of this compound on molecular pathways.
4. Conclusion
Despite vaccine development, it is necessary to identify a potential therapeutic drug to reduce the complications of COVID-19. In this regard, the results of the current study indicate that silymarin as a polyphenolic flavonoid might exert therapeutic effects against COVID-19 through the improvement of the antioxidant system, attenuation of the inflammatory response, and respiratory distress, and enhancement of immune system function. Moreover, silymarin can bind to target proteins of SARS-CoV-2, including main protease, spike glycoprotein, and RNA-dependent RNA-polymerase, leading to the inhibition of viral replication. Furthermore, further clinical trials and human studies are encouraged considering the effects of silymarin in COVID-19 patients.
CRediT authorship contribution statement
The authors’ roles were as follows. AK, VM, NB, S.S, HRN: developed the first hypothesis of the study, searched the data, both authors assessed and extracted data. AK, MK, MV, wrote the draft of the manuscript. HRN: contributed to data collection; AK, MK and MV: provided advice and consultation; HRN: contributed to the final revision of the manuscript.
Funding source
None.
Conflict of interest statement
The authors declare that they have no competing interests.
Data availability
All data generated or analyzed are included in the results of the manuscript.
References
- 1.Costanza G., Paba P., Ciotti M., Ombres D., Di Carlo S., Marcuccilli F., et al. Infection rate of respiratory viruses in the pandemic SARS-CoV-2 period considering symptomatic patients: two years of ongoing observations. Biomolecules. 2022;12(7):987. doi: 10.3390/biom12070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ibrahim C., Hammami S., Ghanmi E., Hassen A. Emerging Human Coronaviruses (SARS-CoV-2) in the Environment Associated with Outbreaks Viral Pandemics. 2022.
- 3.Rayan R.A., Tsagkaris C., Zafar I., Tata A. COVID-19 and SARS-CoV-2. CRC Press; 2022. Epidemiology of COVID-19; pp. 63–78. [Google Scholar]
- 4.Trugilho M.R., Azevedo-Quintanilha I.G., Gesto J.S., Moraes E.C.S., Mandacaru S.C., Campos M.M., et al. Platelet proteome reveals features of cell death, antiviral response and viral replication in covid-19. Cell Death Discov. 2022;8(1):1–11. doi: 10.1038/s41420-022-01122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallo Marin B., Aghagoli G., Lavine K., Yang L., Siff E.J., Chiang S.S., et al. Predictors of COVID‐19 severity: a literature review. Rev. Med. Virol. 2021;31(1):1–10. doi: 10.1002/rmv.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaur R., Singh S., Singh T.G., Sood P., Robert J. Covid-19: pharmacotherapeutic insights on various curative approaches in terms of vulnerability, comorbidities, and vaccination. Inflammopharmacology. 2022:1–21. doi: 10.1007/s10787-021-00904-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrera V.L., Walkey A.J., Nguyen M.Q., Gromisch C.M., Mosaddhegi J.Z., Gromisch M.S., et al. A targetable ‘rogue’neutrophil-subset,[CD11b+ DEspR+] immunotype, is associated with severity and mortality in acute respiratory distress syndrome (ARDS) and COVID-19-ARDS. Sci. Rep. 2022;12(1):1–24. doi: 10.1038/s41598-022-09343-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atici A., Asoglu R., Barman H.A., Tatlisu M.A., Aciksari G., Yilmaz Y., et al. Evaluation of COVID-19 patients according to the survival time. Acta Med. Indones. 2022;54(2):176–189. [PubMed] [Google Scholar]
- 9.Li X., Zhong X., Wang Y., Zeng X., Luo T., Liu Q. Clinical determinants of the severity of COVID-19: a systematic review and meta-analysis. PLoS One. 2021;16(5) doi: 10.1371/journal.pone.0250602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y., Ma Y., Xu Y., Liu J., Li X., Chen Y., et al. Resistance of SARS-CoV-2 Omicron variant to convalescent and CoronaVac vaccine plasma. Emerg. Microbes Infect. 2022;11(1):424–427. doi: 10.1080/22221751.2022.2027219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rivera S.L.R., Cantú J.A.I., Martínez H.R., Osorio E.C. Pathogenesis Coronavirus Disease 2019 (COVID-19): Narrative Literature Review. Biosci. Med.: J. Biomed. Transl. Res. 2022;6(7):2029–2033. [Google Scholar]
- 12.Makaremi S., Asgarzadeh A., Kianfar H., Mohammadnia A., Asghariazar V., Safarzadeh E. The role of IL-1 family of cytokines and receptors in pathogenesis of COVID-19. Inflamm. Res. 2022:1–25. doi: 10.1007/s00011-022-01596-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babajani A., Moeinabadi-Bidgoli K., Niknejad F., Rismanchi H., Shafiee S., Shariatzadeh S., et al. Human placenta-derived amniotic epithelial cells as a new therapeutic hope for COVID-19-associated acute respiratory distress syndrome (ARDS) and systemic inflammation. Stem Cell Res. Ther. 2022;13(1):1–22. doi: 10.1186/s13287-022-02794-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veenith T., Martin H., Le Breuilly M., Whitehouse T., Gao-Smith F., Duggal N., et al. High generation of reactive oxygen species from neutrophils in patients with severe COVID-19. Sci. Rep. 2022;12(1):1–9. doi: 10.1038/s41598-022-13825-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eteraf-Oskouei T., Najafi M. The relationship between the serotonergic system and COVID-19 disease: a review. Heliyon. 2022 doi: 10.1016/j.heliyon.2022.e09544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pidiyar V., Kumraj G., Ahmed K., Ahmed S., Shah S., Verma B., et al. COVID-19 management landscape-a need for an affordable to manufacture safe and efficacious bio-therapeutic and prophylactic for developing countries. Vaccine. 2022 doi: 10.1016/j.vaccine.2022.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sardu C., Marfella R., Prattichizzo F., La Grotta R., Paolisso G., Ceriello A. Effect of hyperglycemia on COVID-19 outcomes: vaccination efficacy, disease severity, and molecular mechanisms. J. Clin. Med. 2022;11(6):1564. doi: 10.3390/jcm11061564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biswas M., Das A., Basu S. Flavonoids: the innocuous agents offering protection against Alz-heimer’s disease through modulation of proinflammatory and apoptotic pathways. Curr. Top. Med. Chem. 2022 doi: 10.2174/1568026622666220330011645. [DOI] [PubMed] [Google Scholar]
- 19.Magiera A., Czerwińska M.E., Owczarek A., Marchelak A., Granica S., Olszewska M.A. Polyphenols and maillard reaction products in dried prunus spinosa fruits: quality aspects and contribution to anti-inflammatory and antioxidant activity in human immune cells ex vivo. Molecules. 2022;27(10):3302. doi: 10.3390/molecules27103302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caponio G.R., Lippolis T., Tutino V., Gigante I., De Nunzio V., Milella R.A., et al. Nutraceuticals: focus on anti-inflammatory, anti-cancer, antioxidant properties in gastrointestinal tract. Antioxidants. 2022;11(7):1274. doi: 10.3390/antiox11071274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dwivedi P.S., Patil V.S., Khanal P., Bhandare V.V., Gurav S., Harish D.R., et al. System biology-based investigation of Silymarin to trace hepatoprotective effect. Comput. Biol. Med. 2022;142 doi: 10.1016/j.compbiomed.2022.105223. [DOI] [PubMed] [Google Scholar]
- 22.Hanafy N.A., El-Kemary M.A. Silymarin/curcumin loaded albumin nanoparticles coated by chitosan as muco-inhalable delivery system observing anti-inflammatory and anti COVID-19 characterizations in oleic acid triggered lung injury and in vitro COVID-19 experiment. Int. J. Biol. Macromol. 2022;198:101–110. doi: 10.1016/j.ijbiomac.2021.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fallah M., Davoodvandi A., Nikmanzar S., Aghili S., Mirazimi S.M.A., Aschner M., et al. Silymarin (milk thistle extract) as a therapeutic agent in gastrointestinal cancer. Biomed. Pharmacother. 2021;142 doi: 10.1016/j.biopha.2021.112024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali S.I., Sheikh W.M., Rather M.A., Venkatesalu V., Muzamil Bashir S., Nabi S.U. Medicinal plants: Treasure for antiviral drug discovery. Phytother. Res. 2021;35(7):3447–3483. doi: 10.1002/ptr.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loutfy S.A., Abdel-Salam A.I., Moatasim Y., Gomaa M.R., Fattah N.F.A., Emam M.H., et al. Antiviral activity of chitosan nanoparticles encapsulating silymarin (Sil–CNPs) against SARS-CoV-2 (in silico and in vitro study) RSC Adv. 2022;12(25):15775–15786. doi: 10.1039/d2ra00905f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bidart G.N., Putkaradze N., Fredslund F., Kjeldsen C., Ruiz A.G., Duus J.Ø., et al. Family 1 Glycosyltransferase UGT706F8 from Zea mays Selectively Catalyzes the Synthesis of Silibinin 7-O-β-d-Glucoside. ACS Sustain. Chem. Eng. 2022;10(16):5078–5083. doi: 10.1021/acssuschemeng.1c07593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pourgholi A., Dadashpour M., Mousapour A., Amandi A.F., Zarghami N. Anticancer potential of silibinin loaded polymeric nanoparticles against breast cancer cells: Insight into the apoptotic genes targets. Asian Pac. J. Cancer Prev.: APJCP. 2021;22(8):2587. doi: 10.31557/APJCP.2021.22.8.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Speciale A., Muscarà C., Molonia M.S., Cimino F., Saija A., Giofrè S.V. Silibinin as potential tool against SARS‐Cov‐2: In silico spike receptor‐binding domain and main protease molecular docking analysis, and in vitro endothelial protective effects. Phytother. Res. 2021;35(8):4616–4625. doi: 10.1002/ptr.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamdy R., Mostafa A., Abo, Shama N.M., Soliman S.S., Fayed B. Comparative evaluation of flavonoids reveals the superiority and promising inhibition activity of silibinin against SARS‐CoV‐2. Phytother. Res. 2022 doi: 10.1002/ptr.7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aguilar‑Lemarroy A., López‑Uribe A., Sánchez‑Corona J., Jave‑suárez L.F. Severe acute respiratory syndrome coronavirus 2 ORF3a induces the expression of ACE2 in oral and pulmonary epithelial cells and the food supplement Vita Deyun® diminishes this effect. Experimental and Therapeutic. Medicine. 2021;21(5):1–8. doi: 10.3892/etm.2021.9916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nieuwenhuizen L., De Groot P.G., Grutters J.C., Biesma D.H. A review of pulmonary coagulopathy in acute lung injury, acute respiratory distress syndrome and pneumonia. Eur. J. Haematol. 2009;82(6):413–425. doi: 10.1111/j.1600-0609.2009.01238.x. [DOI] [PubMed] [Google Scholar]
- 32.Aryan H., Farahani R.H., Chamanara M., Elyasi S., Jaafari M.R., Haddad M., et al. Evaluation of the efficacy of oral nano‐silymarin formulation in hospitalized patients with COVID‐19: A double‐blind placebo‐controlled clinical trial. Phytotherapy Research. [DOI] [PMC free article] [PubMed]
- 33.Aranyó J., Bazan V., Lladós G., Dominguez M.J., Bisbal F., Massanella M., et al. Inappropriate sinus tachycardia in post-COVID-19 syndrome. Sci. Rep. 2022;12(1):1–9. doi: 10.1038/s41598-021-03831-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J., Liu J., Chen Z., Peng H., Zhu C., Feng D., et al. Angiotensin-converting enzyme 2 potentiates SARS-CoV-2 infection by antagonizing type I interferon induction and its down-stream signaling pathway. mSphere. 2022:e00211–e00222. doi: 10.1128/msphere.00211-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kashani N.R., Azadbakht J., Ehteram H., Kashani H.H., Rajabi-Moghadam H., Ahmad E., et al. Molecular and clinical investigation of COVID-19: from pathogenesis and immune responses to novel diagnosis and treatment. Front. Mol. Biosci. 2022:9. doi: 10.3389/fmolb.2022.770775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wielgat P., Narejko K., Car H. SARS-CoV-2 attacks in the brain: focus on the sialome. Cells. 2022;11(9):1458. doi: 10.3390/cells11091458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karimi Shahri M., Niazkar H.R., Rad F. COVID‐19 and hematology findings based on the current evidences: a puzzle with many missing pieces. Int. J. Lab. Hematol. 2021;43(2):160–168. doi: 10.1111/ijlh.13412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adem Ş., Eyupoglu V., Ibrahim I.M., Sarfraz I., Rasul A., Ali M., et al. Multidimensional in silico strategy for identification of natural polyphenols-based SARS-CoV-2 main protease (M(pro)) inhibitors to unveil a hope against COVID-19. Comput. Biol. Med. 2022;145 doi: 10.1016/j.compbiomed.2022.105452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang Y., Rubin L., Peng T., Liu L., Xing X., Lazarovici P., et al. Cytokine storm in COVID-19: from viral infection to immune responses, diagnosis and therapy. Int. J. Biol. Sci. 2022;18(2):459. doi: 10.7150/ijbs.59272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silva M.J.A., Rodrigues Y.C., Lima K.V.B., Lima L.N.G.C. Innate immunity to SARS-CoV-2 infection: A review. Epidemiology & Infection.1–49. [DOI] [PMC free article] [PubMed]
- 41.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clinical Infectious Diseases. 2020. [DOI] [PMC free article] [PubMed]
- 42.Sengupta P., Dutta S., Slama P. Roychoudhury S. COVID-19, oxidative stress, and male reproductive dysfunctions: is vitamin C a potential remedy? Physiol. Res. 2022;71(1):47. doi: 10.33549/physiolres.934827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rotariu D., Babes E.E., Tit D.M., Moisi M., Bustea C., Stoicescu M., et al. Oxidative stress–Complex pathological issues concerning the hallmark of cardiovascular and metabolic disorders. Biomed. Pharmacother. 2022;152 doi: 10.1016/j.biopha.2022.113238. [DOI] [PubMed] [Google Scholar]
- 44.Haftcheshmeh S.M., Abedi M., Mashayekhi K., Mousavi M.J., Navashenaq J.G., Mohammadi A., et al. Berberine as a natural modulator of inflammatory signaling pathways in the immune system: Focus on NF‐κB, JAK/STAT, and MAPK signaling pathways. Phytother. Res. 2022;36(3):1216–1230. doi: 10.1002/ptr.7407. [DOI] [PubMed] [Google Scholar]
- 45.Zaffagni M., Harris J.M., Patop I.L., Pamudurti N.R., Nguyen S., Kadener S. SARS-CoV-2 Nsp14 mediates the effects of viral infection on the host cell transcriptome. Elife. 2022;11 doi: 10.7554/eLife.71945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roingeard P., Eymieux S., Burlaud-Gaillard J., Hourioux C., Patient R., Blanchard E. The double-membrane vesicle (DMV): a virus-induced organelle dedicated to the replication of SARS-CoV-2 and other positive-sense single-stranded RNA viruses. Cell. Mol. Life Sci. 2022;79(8):1–9. doi: 10.1007/s00018-022-04469-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He C., He X., Yang J., Lei H., Hong W., Song X., et al. Spike protein of SARS‐CoV‐2 Omicron (B. 1.1. 529) variant has a reduced ability to induce the immune response. Signal Transduct. Target. Ther. 2022;7(1):1–4. doi: 10.1038/s41392-022-00980-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Falzarano D., De Wit E., Rasmussen A.L., Feldmann F., Okumura A., Scott D.P., et al. Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV–infected rhesus macaques. Nat. Med. 2013;19(10):1313–1317. doi: 10.1038/nm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao W., Wang L., Ju X., Zhao S., Li Z., Su M., et al. The deubiquitinase USP29 Promotes SARS-CoV-2 virulence by preventing proteasome degradation of ORF9b. mBio. 2022:e01300–e01322. doi: 10.1128/mbio.01300-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Al-Kuraishy H.M., Al-Gareeb A.I., Al-Hussaniy H.A., Al-Harcan N.A.H., Alexiou A., Batiha G.E.-S. Neutrophil Extracellular Traps (NETs) and Covid-19: a new frontiers for therapeutic modality. Int. Immunopharmacol. 2022 doi: 10.1016/j.intimp.2021.108516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loyer C., Lapostolle A., Urbina T., Elabbadi A., Lavillegrand J.-R., Chaigneau T., et al. Impairment of neutrophil functions and homeostasis in COVID-19 patients: association with disease severity. Crit. Care. 2022;26(1):1–16. doi: 10.1186/s13054-022-04002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Devi P., Maurya R., Mehta P., Shamim U., Yadav A., Chattopadhyay P., et al. Increased Abundance of Achromobacter xylosoxidans and Bacillus cereus in upper airway transcriptionally active microbiome of COVID-19 mortality patients indicates role of co-infections in disease severity and outcome. Microbiol. Spectr. 2022:e02311–e02321. doi: 10.1128/spectrum.02311-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lamers M.M., Haagmans B.L. SARS-CoV-2 pathogenesis. Nat. Rev. Microbiol. 2022;20(5):270–284. doi: 10.1038/s41579-022-00713-0. [DOI] [PubMed] [Google Scholar]
- 54.Schreiber A., Viemann D., Schöning J., Schloer S., Mecate Zambrano A., Brunotte L., et al. The MEK1/2-inhibitor ATR-002 efficiently blocks SARS-CoV-2 propagation and alleviates pro-inflammatory cytokine/chemokine responses. Cell. Mol. Life Sci. 2022;79(1):1–18. doi: 10.1007/s00018-021-04085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang J.-K., Zhao M.-M., Jin J.-M., Liu S., Bai P., He W., et al. New-onset COVID-19–related diabetes: an early indicator of multi-organ injury and mortally of SARS-CoV-2 infection. Curr. Med. 2022;1(1):1–10. doi: 10.1007/s44194-022-00006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Corchete P. Bioactive Molecules and Medicinal Plants. Springer; 2008. Silybum marianum (L.) Gaertn: the source of silymarin; pp. 123–148. [Google Scholar]
- 57.Pradhan S., Girish C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J. Med. Res. 2006;124(5):491–504. [PubMed] [Google Scholar]
- 58.Karimi G., Vahabzadeh M., Lari P., Rashedinia M., Moshiri M. “Silymarin”, a promising pharmacological agent for treatment of diseases. Iran. J. Basic Med. Sci. 2011;14(4):308. [PMC free article] [PubMed] [Google Scholar]
- 59.Abenavoli L., Izzo A.A., Milić N., Cicala C., Santini A., Capasso R. Milk thistle (Silybum marianum): a concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother. Res. 2018;32(11):2202–2213. doi: 10.1002/ptr.6171. [DOI] [PubMed] [Google Scholar]
- 60.Song J., Choi H. Silymarin efficacy against influenza A virus replication. Phytomedicine. 2011;18(10):832–835. doi: 10.1016/j.phymed.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 61.Gordon A., Hobbs D.A., Bowden D.S., Bailey M.J., Mitchell J., Francis A.J., et al. Effects of Silybum marianum on serum hepatitis C virus RNA, alanine aminotransferase levels and well‐being in patients with chronic hepatitis C. J. Gastroenterol. Hepatol. 2006;21(1):275–280. doi: 10.1111/j.1440-1746.2006.04138.x. [DOI] [PubMed] [Google Scholar]
- 62.Sherman D., Williams R. Liver damage: mechanisms and management. Br. Med. Bull. 1994;50(1):124–138. doi: 10.1093/oxfordjournals.bmb.a072871. [DOI] [PubMed] [Google Scholar]
- 63.Hsieh C., Macatonia S., Tripp C., Wolf S., O'Garra A., Murphy K. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260(5107):547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 64.Scott P. IL-12: initiation cytokine for cell-mediated immunity. Science. 1993;260(5107):496–498. doi: 10.1126/science.8097337. [DOI] [PubMed] [Google Scholar]
- 65.Manetti R., Parronchi P., Giudizi M.G., Piccinni M., Maggi E., Trinchieri G., et al. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J. Exp. Med. 1993;177(4):1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Katiyar S.K. Treatment of silymarin, a plant flavonoid, prevents ultraviolet light-induced immune suppression and oxidative stress in mouse skin. Int. J. Oncol. 2002;21(6):1213–1222. [PubMed] [Google Scholar]
- 67.Meeran S.M., Katiyar S., Elmets C.A., Katiyar S.K. Silymarin inhibits UV radiation-induced immunosuppression through augmentation of interleukin-12 in mice. Mol. Cancer Ther. 2006;5(7):1660–1668. doi: 10.1158/1535-7163.MCT-06-0095. [DOI] [PubMed] [Google Scholar]
- 68.Meroni P., Barcellini W., Borghi M., Vismara A., Ferraro G., Ciani D., et al. Silybin inhibition of human T-lymphocyte activation. Int. J. Tissue React. 1988;10(3):177–181. [PubMed] [Google Scholar]
- 69.Valková V., Ďúranová H., Bilčíková J., Habán M. MILK THISTLE (Silybum marianum): a valuable medicinal plant with several therapeutic purposes. J. Microbiol., Biotechnol. Food Sci. 2020;9(5):836–843. [Google Scholar]
- 70.Vaid M., Katiyar S.K. Molecular mechanisms of inhibition of photocarcinogenesis by silymarin, a phytochemical from milk thistle (Silybum marianum L. Gaertn.) Int. J. Oncol. 2010;36(5):1053–1060. doi: 10.3892/ijo_00000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Valková V., Ďúranová H., Bilčíková J., Habán M. Milk thistle (Silybum marianum): a valuable medicinal plant with several therapeutic purposes. Journal of Microbiology. Biotechnol. Food Sci. 2021;2021:836–843. [Google Scholar]
- 72.Elrashdy F., Aljaddawi A.A., Redwan E.M., Uversky V.N. On the potential role of exosomes in the COVID-19 reinfection/reactivation opportunity. J. Biomol. Struct. Dyn. 2020:1–12. doi: 10.1080/07391102.2020.1790426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang F., Nie J., Wang H., Zhao Q., Xiong Y., Deng L., et al. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J. Infect. Dis. 2020;221(11):1762–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020;130(5) doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lovelace E.S., Maurice N.J., Miller H.W., Slichter C.K., Harrington R., Magaret A., et al. Silymarin suppresses basal and stimulus-induced activation, exhaustion, differentiation, and inflammatory markers in primary human immune cells. PloS One. 2017;12(2) doi: 10.1371/journal.pone.0171139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chiappetta S., Sharma A.M., Bottino V., Stier C. COVID-19 and the role of chronic inflammation in patients with obesity. Int. J. Obes. 2020:1–3. doi: 10.1038/s41366-020-0597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feng X., Li S., Sun Q., Zhu J., Chen B., Xiong M., et al. Immune-inflammatory parameters in COVID-19 cases: a systematic review and meta-analysis. Front. Med. 2020;7:301. doi: 10.3389/fmed.2020.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Derouiche S. Oxidative stress associated with SARS-Cov-2 (COVID-19) increases the severity of the lung disease-a systematic review. J. Infect. Dis. Epidemiol. 2020;6:121. [Google Scholar]
- 79.Zhang D., Guo R., Lei L., Liu H., Wang Y., Wang Y., et al. COVID‐19 infection induces readily detectable morphologic and inflammation‐related phenotypic changes in peripheral blood monocytes. J. Leukoc. Biol. 2020 doi: 10.1002/JLB.4HI0720-470R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sharafkhaneh A., Hanania N.A., Kim V. Pathogenesis of emphysema: from the bench to the bedside. Proc. Am. Thorac. Soc. 2008;5(4):475–477. doi: 10.1513/pats.200708-126ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Esmaeil N., Anaraki S.B., Gharagozloo M., Moayedi B. Silymarin impacts on immune system as an immunomodulator: One key for many locks. Int. Immunopharmacol. 2017;50:194–201. doi: 10.1016/j.intimp.2017.06.030. [DOI] [PubMed] [Google Scholar]
- 82.Gharagozloo M., Jafari S., Esmaeil N., Javid E.N., Bagherpour B., Rezaei A. Immunosuppressive effect of silymarin on mitogen‐activated protein kinase signalling pathway: the impact on t cell proliferation and cytokine production. Basic Clin. Pharmacol. Toxicol. 2013;113(3):209–214. doi: 10.1111/bcpt.12088. [DOI] [PubMed] [Google Scholar]
- 83.Kim E.J., Lee M.Y., Jeon Y.J. Silymarin inhibits morphological changes in LPS-stimulated macrophages by blocking NF-κB pathway. Korean J. Physiol. Pharmacol. 2015;19(3):211–218. doi: 10.4196/kjpp.2015.19.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li D., Hu J., Wang T., Wen F. Silymarin attenuates cigarette smoke extract-induced inflammation via autophagy and Erk/p38 mapk pathway in human bronchial epithelial cells. B29 Autophagy: Mechanisms Therapeutic Opportunities: Am. Thorac. Soc. 2017:A3125. [Google Scholar]
- 85.Ranjbar N., Saravani R., Faezizadeh Z. Silymarin inhibits Toll-like receptor 8 gene expression and apoptosis in Ramos cancer cell line. Avicenna J. Phytomedicine. 2020;10(2):161. [PMC free article] [PubMed] [Google Scholar]
- 86.Akbari-Kordkheyli V., Abbaszadeh-Goudarzi K., Nejati-Laskokalayeh M., Zarpou S., Khonakdar-Tarsi A. The protective effects of silymarin on ischemia-reperfusion injuries: a mechanistic review. Iran. J. Basic Med. Sci. 2019;22(9):968. doi: 10.22038/ijbms.2019.34284.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Surai P.F. Silymarin as a natural antioxidant: an overview of the current evidence and perspectives. Antioxidants. 2015;4(1):204–247. doi: 10.3390/antiox4010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Milić N., Milošević N., Suvajdžić L., Žarkov M., Abenavoli L. New therapeutic potentials of milk thistle (Silybum marianum) Nat. Prod. Commun. 2013;8(12) 1934578X1300801236. [PubMed] [Google Scholar]
- 89.Darvishi‐Khezri H., Salehifar E., Kosaryan M., Karami H., Mahdavi M., Alipour A., et al. Iron‐chelating effect of silymarin in patients with β‐thalassemia major: a crossover randomised control trial. Phytother. Res. 2018;32(3):496–503. doi: 10.1002/ptr.5995. [DOI] [PubMed] [Google Scholar]
- 90.Abenavoli L., Capasso R., Milic N., Capasso F. Milk thistle in liver diseases: past, present, future. Phytother. Res. 2010;24(10):1423–1432. doi: 10.1002/ptr.3207. [DOI] [PubMed] [Google Scholar]
- 91.Liu C.-H., Jassey A., Hsu H.-Y., Lin L.-T. Antiviral activities of silymarin and derivatives. Molecules. 2019;24(8):1552. doi: 10.3390/molecules24081552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Polyak S.J., Morishima C., Shuhart M.C., Wang C.C., Liu Y., Lee D.Y.W. Inhibition of T-cell inflammatory cytokines, hepatocyte NF-κB signaling, and HCV infection by standardized silymarin. Gastroenterology. 2007;132(5):1925–1936. doi: 10.1053/j.gastro.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 93.Wagoner J., Negash A., Kane O.J., Martinez L.E., Nahmias Y., Bourne N., et al. Multiple effects of silymarin on the hepatitis C virus lifecycle. Hepatology. 2010;51(6):1912–1921. doi: 10.1002/hep.23587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ferenci P., Beinhardt S. Silibinin: an old drug in the high tech era of liver transplantation. J. Hepatol. 2013;58(3):409–411. doi: 10.1016/j.jhep.2012.11.030. [DOI] [PubMed] [Google Scholar]
- 95.Maier I., Wu G.Y. Hepatitis C and HIV co-infection: a review. World J. Gastroenterol. 2002;8(4):577. doi: 10.3748/wjg.v8.i4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meissner E.G. Update in HIV/HCV co-infection in the direct acting antiviral era. Curr. Opin. Gastroenterol. 2017;33(3):120. doi: 10.1097/MOG.0000000000000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu Y.-F., Fu S.-L., Kao C.-H., Yang C.-W., Lin C.-H., Hsu M.-T., et al. Chemopreventive effect of silymarin on liver pathology in HBV X protein transgenic mice. Cancer Res. 2008;68(6):2033–2042. doi: 10.1158/0008-5472.CAN-07-2450. [DOI] [PubMed] [Google Scholar]
- 98.Gabbay E., Zigmond E., Pappo O., Hemed N., Rowe M., Zabrecky G., et al. Antioxidant therapy for chronic hepatitis C after failure of interferon: results of phase II randomized, double-blind placebo controlled clinical trial. World J. Gastroenterol.: WJG. 2007;13(40):5317. doi: 10.3748/wjg.v13.i40.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Qaddir I., Rasool N., Hussain W., Mahmood S. Computer-aided analysis of phytochemicals as potential dengue virus inhibitors based on molecular docking, ADMET and DFT studies. J. Vector borne Dis. 2017;54(3):255. doi: 10.4103/0972-9062.217617. [DOI] [PubMed] [Google Scholar]
- 100.Lani R., Hassandarvish P., Chiam C.W., Moghaddam E., Chu J.J.H., Rausalu K., et al. Antiviral activity of silymarin against chikungunya virus. Sci. Rep. 2015;5:11421. doi: 10.1038/srep11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Camini F.C., da Silva T.F., da Silva Caetano C.C., Almeida L.T., Ferraz A.C., Vitoreti V.M.A., et al. Antiviral activity of silymarin against Mayaro virus and protective effect in virus-induced oxidative stress. Antivir. Res. 2018;158:8–12. doi: 10.1016/j.antiviral.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 102.Braun D.L., Rauch A., Aouri M., Durisch N., Eberhard N., Anagnostopoulos A., et al. A lead-in with silibinin prior to triple-therapy translates into favorable treatment outcomes in difficult-to-treat HIV/hepatitis C coinfected patients. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0133028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dai J.-P., Wu L.-Q., Li R., Zhao X.-F., Wan Q.-Y., Chen X.-X., et al. Identification of 23-(s)-2-amino-3-phenylpropanoyl-silybin as an antiviral agent for influenza A virus infection in vitro and in vivo. Antimicrob. Agents Chemother. 2013;57(9):4433–4443. doi: 10.1128/AAC.00759-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McClure J., Lovelace E.S., Elahi S., Maurice N.J., Wagoner J., Dragavon J., et al. Silibinin inhibits HIV-1 infection by reducing cellular activation and proliferation. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0041832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ahmed–Belkacem A., Ahnou N., Barbotte L., Wychowski C., Pallier C., Brillet R., et al. Silibinin and related compounds are direct inhibitors of hepatitis C virus RNA-dependent RNA polymerase. Gastroenterology. 2010;138(3):1112–1122. doi: 10.1053/j.gastro.2009.11.053. [DOI] [PubMed] [Google Scholar]
- 106.Torres M., Rodríguez-Serrano F., Rosario D.J., Rodríguez-Pérez F., Toro D.H. Does Silybum marianum play a role in the treatment of chronic hepatitis C? Puerto Rico Health Sci. J. 2013;23:2. [PubMed] [Google Scholar]
- 107.Yakoot M., Salem A. Spirulina platensis versus silymarin in the treatment of chronic hepatitis C virus infection. A pilot randomized, comparative clinical trial. BMC Gastroenterol. 2012;12(1):32. doi: 10.1186/1471-230X-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Biermer M., Schlosser B., Fülöp B., van Bömmel F., Brodzinski A., Heyne R., et al. High‐dose silibinin rescue treatment for HCV‐infected patients showing suboptimal virologic response to standard combination therapy. J. Viral Hepat. 2012;19(8):547–553. doi: 10.1111/j.1365-2893.2011.01572.x. [DOI] [PubMed] [Google Scholar]
- 109.Adeyemo O., Doi H., Rajender Reddy K., Kaplan D.E. Investigators ST. Impact of oral silymarin on virus‐and non‐virus‐specific T‐cell responses in chronic hepatitis C infection. J. Viral Hepat. 2013;20(7):453–462. doi: 10.1111/jvh.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Patel C.N., Kumar S.P., Pandya H.A., Rawal R.M. Identification of potential inhibitors of coronavirus hemagglutinin-esterase using molecular docking, molecular dynamics simulation and binding free energy calculation. Mol. Divers. 2021;25(1):421–433. doi: 10.1007/s11030-020-10135-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z., et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The experience of clinical immunologists from China. Clin. Immunol. 2020;108393 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mariño Z., Crespo G., D’Amato M., Brambilla N., Giacovelli G., Rovati L., et al. Intravenous silibinin monotherapy shows significant antiviral activity in HCV-infected patients in the peri-transplantation period. J. Hepatol. 2013;58(3):415–420. doi: 10.1016/j.jhep.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 113.Latha N., Pandit M. In silico studies reveal potential antiviral activity of phytochemicals from medicinal plants for the treatment of COVID-19 infection. 2020 [Google Scholar]
- 114.Huang J., Song W., Huang H., Sun Q. Pharmacological therapeutics targeting RNA-dependent RNA polymerase, proteinase and spike protein: from mechanistic studies to clinical trials for COVID-19. J. Clin. Med. 2020;9(4):1131. doi: 10.3390/jcm9041131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Khan S.S. The Central Role of PAI-1 in COVID-19: thrombosis and beyond. Am. Thorac. Soc. 2021:238–240. doi: 10.1165/rcmb.2021-0208ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed are included in the results of the manuscript.