Figure 7.
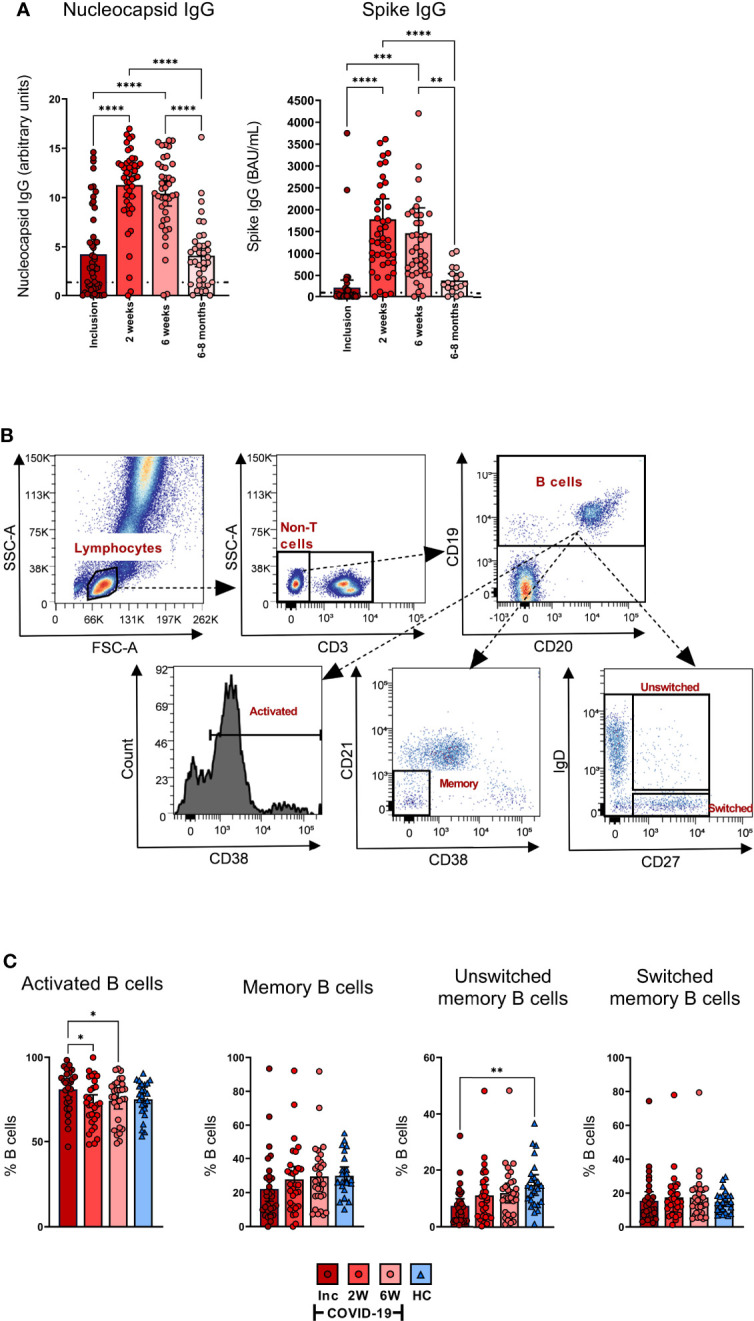
All COVID-19 patients develop SARS-CoV2-specific antibody responses and have activated B cells early in the disease. Sera were collected from COVID-19 patients that required hospitalization (N=46) and healthy donors (N=31). (A) The levels of nucleocapsid antibodies (Units, 0.8-1.4 borderline positive and > 1.4 for positive result) (left) and spike antibodies (BAU/ml, ≥ 30 for positive result) (right) were measured in sera from inclusion up to 6-8 months. (B) Representative gating to determine B cell proportions and activation states using CD3, CD19, CD20, CD21, CD38, IgD, and CD27. (C) The B cell populations measured by flow cytometry as percentage out of the CD19+ B cells; CD19+CD38+ B cells (activated B cells), CD19+CD27+ B cells (memory B cells), CD19+CD27+IgD+ B cells (unswitched B cells), and CD19+CD27+IgD- B cells (Switched B cells). Data is represented as mean with 95% Cl, with significance of *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001, determined using Brown-Forsythe and Welch ANOVA tests. Inc = Inclusion in study at the hospital, 2W = 2 weeks, 6W = 6 weeks, HC = healthy control.
