Figure 6.
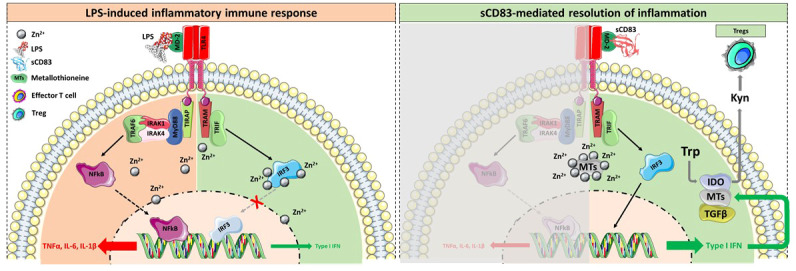
Graphical summary of the sCD83-induced regulatory mechanism. (Upper side) Binding of LPS to the TLR4/MD-2 complex results in the activation of the MyD88 signaling cascade, the translocation of NFκB into the nucleus, and the subsequent expression of pro-inflammatory cytokines. Concomitantly, TLR4 stimulation leads to activation-induced Zn2+ influx into the cytosol, a crucial event for the dissociation of NFκB from its inhibitor, thus facilitating its translocation into the nucleus. Moreover, Zn2+ potently inhibited the TRIF-mediated anti-inflammatory signaling pathway by blocking the IRF3 activity. (Lower side) In sharp contrast, the binding of sCD83 to the TLR4/MD-2 complex fosters the resolution of inflammation in a TRIF-dependent manner. In addition, sCD83 strongly induces the expression of MTs, which bind free Zn2+, thereby promoting the translocation of IRF3 into the nucleus and the subsequent induction of type I IFN. Type I IFNs are potent inducers of IDO and TGF, both shown to be crucial for the sCD83-induced differentiation of Tregs and the long term-resolution of inflammation. Herein IDO converts the amino acid tryptophan (Trp), which is indispensable for effector T cell proliferation and survival, into Kynurenine (Kyn), a potent inducer of Tregs via the Ahr pathway. In addition, type I IFNs further boost the expression of MTs and thus stabilize the TRIF-associated resolving pathway over the pro-inflammatory MyD88 cascade.
