Abstract
Introduction
Heart failure (HF) is a severe and terminal stage of various heart diseases. Left ventricular assist devices (LVADs) are relatively mature and have contributed to the treatment of end-stage HF. Ventricular arrhythmia (VA) is a common complication after LVAD implantation, including ventricular tachycardia and ventricular fibrillation, both of which may cause abnormal circulation.
Methods
A literature search was conducted in the PubMed database, “Ventricular Arrhythmia” OR “VA” OR “Arrhythmia” OR “Ventricular Tachycardia,” OR “Ventricular Fibrillation” AND “LVAD” OR “Left Ventricular Assist Device” OR “Heart Assist Device” as either keywords or MeSH terms, the authors screened the titles and abstracts of the articles. Eventually, 12 original research articles were retrieved.
Results
The 0.83 [95% CI: 0.77, 0.89] of patients were male. A whole of 53% [95% CI: 0.25, 0.81] of VA patients had a history of atrial fibrillation and 61% [95% CI: 0.52, 0.69] had a history of VA. 39% [95% CI: 0.29, 0.49] of the participants had no prior history of VA and experienced new VA following CF-LVAD implantation. Following CF-LVAD implantation, 59% [95% CI: 0.51, 0.67] of patients developed early VA (VA ≤30 days). The 30-day mortality rate of patients was 4% [95% CI: 0.01, 0.07]. And overall mortality was 28% [95% CI: 0.15, 0.41]. The reported incidence of VA after LVAD implantation is not identical in different medical centers and ranges from 20% to 60%. The mechanism of VA after LVAD implantation is summarized as primary cardiomyopathy-related, device mechanical stimulation, myocardial scarring, ventricular displacement, electrolyte regulation, and other processes.
Conclusions
A preoperative VA history is considered a predictor of VA following LVAD implantation in most studies. Multiple mechanisms and factors, such as prevention of “suction events,” ablation, and implantable cardioverter defibrillator, should be considered for the prevention and treatment of postoperative VA in patients requiring long-term VAD treatment. This study provides a reference for the clinical application of LAVD and the prevention of postoperative VA after LVAD implantation. Future multicenter prospective studies with uniform patient follow-up are needed to screen for additional potential risk factors and predictors. These studies will help to define the incidence rate of VA after LAVD implantation. As a result, we provide guidance for the selection of preventive intervention.
Keywords: Left ventricular assist device, Ventricular arrhythmia
Introduction
Heart failure (HF) is a severe and terminal stage of various heart diseases. The prevalence of HF is steadily increasing. More importantly, HF is a disease that consumes significant health care resources, causes noteworthy morbidity and death, and has an important effect on the quality of life [1]. HF is a global health care epidemic that affects approximately 26 million people globally and causes over 1 million hospitalizations in both the USA and Europe each year [2]. Despite significant advances in the pharmacological treatment of HF, heart transplantation remains the optimal treatment for patients with severe or end-stage HF. However, the use of heart transplantation is limited due to factors such as a lack of donors.
Therefore, mechanical circulatory assist devices, as represented by ventricular assist devices (VADs), have gradually become the method of choice during the transitional period of heart transplantation and even an alternative to the long-term treatment of end-stage HF [3]. Among these devices, the left ventricular assist device (LVAD) is relatively mature and has contributed to the treatment of end-stage HF. The incidence of ventricular arrhythmia (VA) after LVAD implantation ranges from 20% to 60% [4]. Ventricular tachycardia (VT) and ventricular fibrillation (VF), both of which may cause loss of perfusion of systemic circulation and pulmonary circulation, low cardiac output, and suspended vital tissue and organ perfusion [5]. Although clinical symptoms of LVAD-assisted patients may be indistinct in a short time, persistent VA may lead to right heart dysfunction, which affects circulating flow and eventually leads to hemodynamic compromise [6, 7, 8]. We aimed to summarize the characteristics of VA after LAVD implantation, perioperative management strategies, and guidance regarding methods for out-of-hospital prevention by reviewing recent case reports, epidemiological studies, risk factors, and prevention and treatment strategies for VA after LVAD implantation and to provide a reference for the clinical application of LAVD and the prevention of postoperative VA after LVAD implantation.
Materials and Methods
Literature Search Strategy
Complete electronic searches were performed in September 2021 by using PubMed. To achieve the maximum sensitiveness of the search strategy, combined terms were used: “Ventricular Arrhythmia” OR “VA” OR “Arrhythmia” OR “Ventricular Tachycardia,” OR “Ventricular Fibrillation” AND “LVAD” OR “Left Ventricular Assist Device” OR “Heart Assist Device” as either keywords or MeSH terms in PubMed. All qualifying references were combed through for additional possibly relevant research, which was then appraised using the inclusion and exclusion criteria.
Selection Criteria
All English-language publications with data on patients over the age of 18 who developed VA after LVAD implantation were examined for inclusion. Articles were omitted if they did not include the information about patients who developed VA after receiving LVAD. Only the most complete reports with the longest follow-up term were selected for quantitative assessment when institutions published duplicate studies with overlapping data on patients and follow-up periods. Studies that were not published in English and did not include human participants were omitted. Also omitted were abstracts, case reports, conference presentations, editorials, reviews, and expert opinions.
Definition of VA
VA was defined as sustained (>30 s) VT or any episode of VF. In this study, we only focused on patients who developed VA after CF-LVAD implantation.
Data Extraction and Critical Appraisal
Two reviewers collected data from article texts, tables, and figures. Discussion and consensus were used to address inconsistencies between the two reviewers.
Statistical Analysis
All analyses were performed with the Review Manager (RevMan) (computer program). Version 5.4 (The Cochrane Collaboration, 2020). Heterogeneity was evaluated using the I2 test. p values <0.05 were considered statistically significant.
Results
Study Characteristics
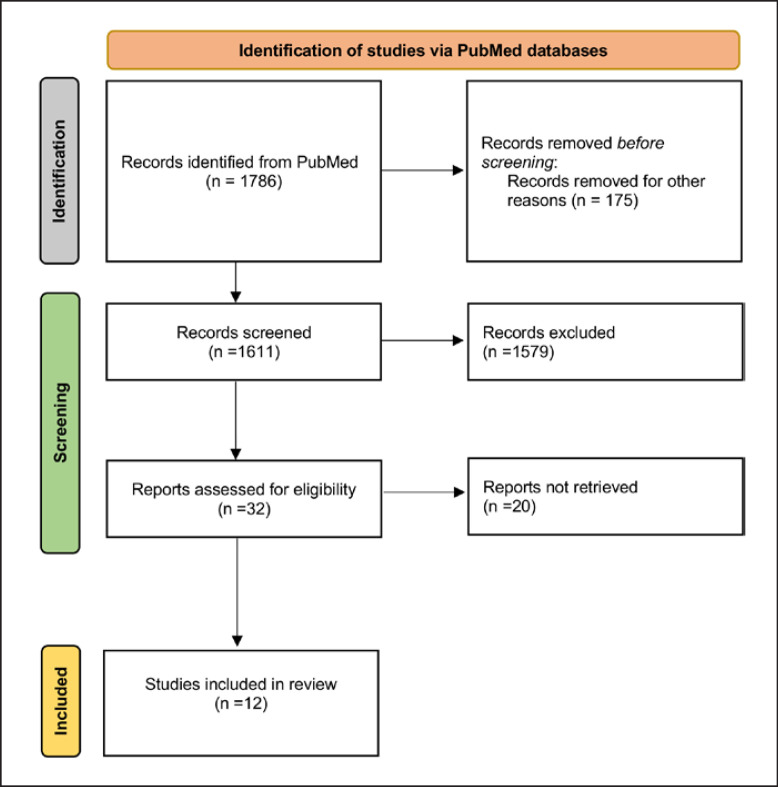
In all, 1,786 records published were found in the literature, and eventually, 12 studies were included in the analysis after applying the inclusion and exclusion criteria. All of the studies were retrospective or prospective studies that investigated VA occurred after LVAD implantation. A manual search of references did not yield further studies. A PRISMA flow diagram displaying the entire search strategy is shown in Figure 1, and a thorough overview of the papers used for analysis is provided in Table 1. This research included a total of 456 individuals who suffered VA after receiving LVAD.
Fig. 1.
PRISMA schematic of the search strategy. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1.
| Author | Year published |
Journal | Study date | Study institution | Type of study | LVAD patients, n | VA patients, n |
Type of LVAD | Key findings |
|---|---|---|---|---|---|---|---|---|---|
| Andersen et al. [18] |
2009 | J Heart Lung Transplant | March 2006 to July 2008 | The Heart Centre, Rigshospitalet, Copenhagen, Denmark | Retrospective | 23 | 12 | HMII (Thoratec, Pleasanton, CA, USA) | About 50% of patients suffered at least an episode of VA after CF-LVAD implantation and no clear predictors be defined. A quarter of patients had significant hemodynamic instability with VA occurred which led the authors to advise to consider performing a prophylactic ICD in CF-LVAD patients |
|
| |||||||||
| Bedi et al. [21] | 2007 | Am J Cardiol | January 1987 to June 2001 | University of Pittsburgh Medical Center, PA, USA | Retrospective | 111 | 24 | Unknown | LVAD patients with ischemic HF history are more frequent occurring VA, and their occurrence is associated with greater mortality |
|
| |||||||||
| Boudghène-Stambouli et al. [61] | 2014 | J Interv Card Electrophysiol | June 2007 to August 2012 | Lille University Hospital, Lille, France | Retrospective | 26 | 11 | HMII (Thoratec, Pleasanton, CA, USA) | About 50% of CF-LVAD implantation patients experienced VA, and prior VA is the strongest predictor |
|
| |||||||||
| Efimova et al. [62] |
2017 | Heart Rhythm | May 2011 to December 2013 | Heart Center Leipzig, Leipzig, Germany | Prospectively | 98 | 48 | HMII (Thoratec, Pleasanton, CA, USA) or Heartware (Heartware, Sydney, NSW, Australia) | Pre-LVAD VA and atrial fibrillation were predictive of VA after CF-LVAD. The occurrence of VA in LVAD implantation patients is greatly high |
|
| |||||||||
| Enriquez et al. [63] |
2013 | Circ Arrhythm Electrophysiol | June 2008 to April 2012 | Mount Sinai Medical Center, NY, USA | Retrospective | 106 | 37 | HMII (Thoratec, Pleasanton, CA, USA) | VA occurs commonly in CF-LVAD patients but VAs are not associated with a worse prognosis, and simultaneous use ICDs may not provide a survival benefit |
|
| |||||||||
| Greet et al. [67] | 2018 | JACC Clin Electrophysiol | January 2000 to April 2015 | Baylor College of Medicine Houston, TX, USA | Retrospective | 517 | 133 | HMII (Thoratec, Pleasanton, CA) or Heartware (Heartware, Sydney, Australia) or other CFLVAD | VA occur in 30 days after CF-LVAD implantation are related to increased mortality. Prior cardiac surgery and VT storm are predictors of early VA |
|
| |||||||||
| Kumar et al. [65] |
2019 | J Interv Card Electrophysiol | January 2008 to March 2018 | Hartford Hospital, Hartford, CT, USA | Retrospective | 157 | 48 | HMII II (Thoratec, Pleasanton, CA, USA) or Heartware (Heartware, Sydney, NSW, Australia) | VA is common in CF-LVAD patients and pre-LVAD VA is a predictor of post-LVAD VA. Neither VA nor ICD shocks are correlated with mortality |
|
| |||||||||
| Moss et al. [54] | 2017 | JACC Clin Electrophysiol | July 2010 to April 2016 | University of Chicago Medicine, Chicago, IL, USA | Retrospective | 21 | 21 | HMII (Thoratec, Pleasanton, CA, USA) or Heartware (Heartware, Sydney, NSW, Australia) | Ablation as destination therapy in post-LVAD patients and freedom from recurrent VT and ICD shocks were related to improved 1-year survival |
|
| |||||||||
| Oswald et al. [40] |
2010 | Eur J Heart Fail | July 2005 to October 2008 | Hannover Medical School, Hannover, Germany | Prospective | 61 | 21 | HMII II (Thoratec, Pleasanton, CA, USA) or Heartware (Heartware, Sydney, NSW, Australia) | ICD therapy is safe and effective in LVAD patients. Prior VA greatly predicts post-LVAD VA and further use of ICD |
|
| |||||||||
| Raasch et al. [45] |
2012 | Am Heart J | January 2006 to February 2011 | University of North Carolina, Chapel Hill, NC, USA | Retrospective | 61 | 26 | HMII (Thoratec Corp, Pleasanton, CA) or Jarvik2000 (Jarvik Heart Inc, New York, NY, USA) | VA occurs commonly in LVAD patients but did not significantly impact survival or transplantation rates |
| Sacher et al. [51] | 2015 | Circ Arrhythm Electrophysiol | 2009–2014 | Bordeaux University, Bordeaux, France Brigham and Women Hospital, Boston, MA, USA Hospital of the University of Pennsylvania, Philadelphia, PA, USA University of Wisconsin Hospital, Madison, Wl, USA University of Miami Medical Center, Miami, FL, USA Institute for Clinical and Experimental Medicine, Prague, Czech Republic Medical College of Virginia/Virginia Commonwealth University School of Medicine, Richmond, VA, USA Richmond CHU de Toulouse, Toulouse, France Mount Sinai Hospital, New York, NY, USA |
Retrospective | 34 | 34 | HMII (Thoratec, Pleasanton, CA, USA) | All patients who occur VT after LVAD implantation have a history of VT before LVAD implantation. Pre-LVAD VA predict post-LVAD VA |
|
| |||||||||
| Yoruk et al. [64] |
2016 | Heart Rhythm | January 2005 to September 2013 | University of Rochester Medical Center, Rochester, NY, USA | Retrospective | 149 | 41 | HMII (Thoratec, Pleasanton, CA, USA) | History of VA and AF strongly predict post-LVAD VA which is associated with an increased risk of all-cause mortality |
Baseline Demographics
Baseline demographics of patients who developed VA are shown in Table 2. The 0.83 [95% CI: 0.77, 0.89] of patients were male. A total of 0.40 [95% CI: 0.32, 0.49] of patients had diabetes mellitus history and 0.58 [95% CI: 0.52, 0.64] had hypertension history. HeartMate II LVAD was used by 0.85 [95% CI: 0.78, 0.93] patients, whereas HeartWare HVAD was used by just 0.04 [95% CI: 0.01, 0.06]. 3% [95% CI: 0.00, 0.05] of patients used the other type of device model. In 58% [95% CI: 0.45, 0.70] of patients, the LVAD was used as a bridge to transplantation, and in 42% [95% CI: 0.29, 0.54] of cases, it was used as destination therapy.
Table 2.
Baseline characteristics of patients who experienced VA after LVAD implantation
| Pooled value [95% CI] | Studies, n | Patients, n (N or n/N) | I2, % | |
|---|---|---|---|---|
| Male, % | 83 [0.77, 0.89] | 10 | 322/398 | 60 |
| Past medical history, % | ||||
| Diabetes mellitus | 40 [0.32, 0.49] | 4 | 105/248 | 40 |
| Hypertension | 58 [0.52, 0.64] | 4 | 144/248 | 0 |
| HF etiology, % | ||||
| Ischemic | 53 [0.44, 0.62] | 10 | 156/302 | 60 |
| Nonischemic | 47 [0.38, 0.56] | 10 | 146/302 | 60 |
| Pump type, % | ||||
| HeartMate II LVAD | 85 [0.78, 0.93] | 9 | 300/390 | 95 |
| HeartWare HVAD | 4 [.01, 0.06] | 9 | 28/390 | 69 |
| Other CF-LVAD | 3 [0.00, 0.05] | 11 | 35/432 | 76 |
| Indication for CF-LVAD, % | ||||
| Bridge to transplant | 58 [0.45, 0.70] | 5 | 170/285 | 77 |
| Destination therapy | 42 [0.29, 0.54] | 5 | 113/285 | 76 |
Characteristics of VA
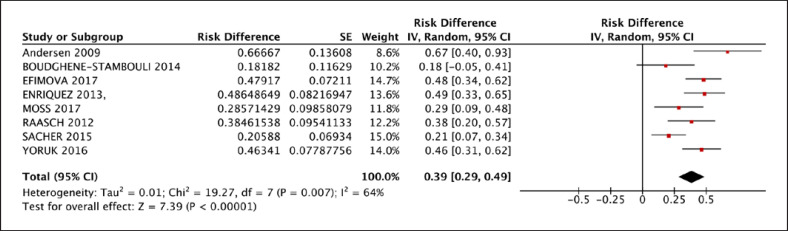
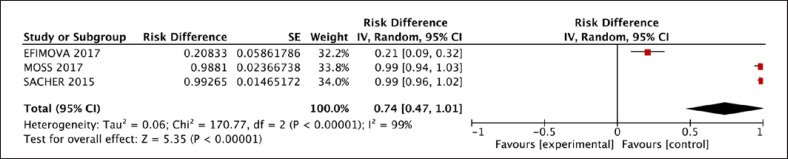
A total of 34% [95% CI: 0.28, 0.40] of patients who received LVAD had VA during LVAD therapy. A whole of 53% [95% CI: 0.25, 0.81] of VA patients had a history of atrial fibrillation and 61% [95% CI: 0.52, 0.69] had a history of VA, which is shown in Table 3. Thirty-nine percent [95% CI: 0.29, 0.49] of the participants had no prior history of VA and experienced new VA following CF-LVAD implantation (Fig. 2). Following CF-LVAD implantation, 59% [95% CI: 0.51, 0.67] of patients developed early VA (VA ≤30 days). For treatment of VA, anti-tachycardiac pacing (ATP) was given to 0.27% [95% CI: 0.04, 0.49], cardioversion was given in 69% [95% CI: 0.57, 0.82], and ablation was performed in 74% [95% CI: 0.47, 1.01] of patients (Fig. 3).
Table 3.
Characteristics of VA after LVAD implantation
| Pooled value [95% CI] | Studies, n | Patients, n (N or n/N) | I2,% | |
|---|---|---|---|---|
| History of arrhythmias, % | ||||
| Atrial fibrillation | 53 [0.25, 0.81] | 4 | 118/311 | 96 |
| VA | 61 [0.52, 0.69] | 9 | 164/278 | 59 |
| New VA after LVAD, % | 39 [0.29, 0.49] | 8 | 93/230 | 64 |
| VA ≤30 days, % | 59 [0.51, 0.67] | 7 | 177/311 | 43 |
| Treatment for VA, % | ||||
| Anti-tachycardial pacing | 27 [0.04, 0.49] | 3 | 12/49 | 69 |
| Cardioversion | 69 [0.57, 0.82] | 3 | 34/49 | 0 |
| Ablation | 74 [0.47, 1.01] | 3 | 65/103 | 99 |
Fig. 2.
New VA rate reported for patients who experienced VA after LVAD implantation.
Fig. 3.
Ablation rate reported in patients for treatment of VA after LVAD implantation.
Outcomes of Patients with Post-LVAD VA
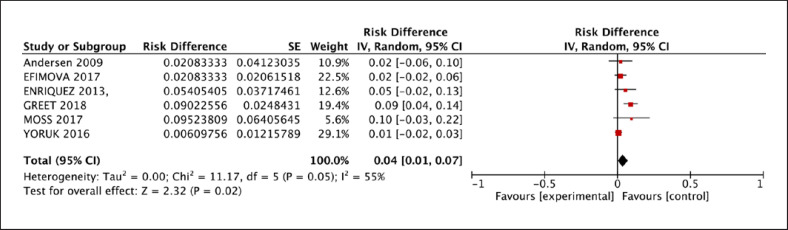
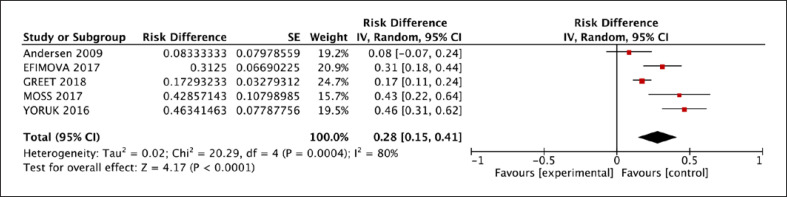
The 30-day mortality of patients was 4% [95% CI: 0.01, 0.07] (Fig. 4), and overall mortality was 28% [95% CI: 0.15, 0.41]. 25% [95% CI: 0.13, 0.36] of VA patients after LVAD implantation were transplanted eventually (Fig. 5). Postoperative outcomes are outlined in Table 4.
Fig. 4.
Early (≤30 days) mortality rate reported for patients who experienced VA after LVAD implantation.
Fig. 5.
Overall mortality rate reported for patients who experienced VA after LVAD implantation.
Table 4.
Outcomes of CF-LVAD patients who experienced VA after implantation
| Pooled value [95% CI] |
Studies, n | Patients, n (N or n/N) |
I2, % |
|
|---|---|---|---|---|
| Mortality, 30 days, % | 4 [0.01, 0.07] | 6 | 17/292 | 55 |
| Mortality, overall, % | 28 [0.15, 0.41] | 5 | 67/255 | 80 |
| Transplanted, % | 25 [0.13, 0.36] | 2 | 14/55 | 5 |
Case Reports and Epidemiological Studies
VA is more common within the first 30 days after LVAD implantation, and approximately one-third of patients may progress to terminal VA [9, 10, 11]. LVADs do not rely on heart rate to sustain cardiac output directly, and thus symptoms are frequently milder and hemodynamics are more stable in LVAD-assisted patients with short-term VA [4]. A case of VA with HeartWare® was reported by Smith and Moak [12]. A 50-year-old female patient was sent to the hospital with a low flow alert on the device and periodic dizziness 6 years after LAVD implantation. A physical examination revealed VF, which was treated with electrical cardioversion. Other similar reports [13, 14, 15] also documented that the patients' circulation was relatively stable and clinical symptoms were modest. Long-term VA persistence or recurrence following LVAD implantation is linked to hemodynamic failure [11]. Multiple recurrences of VF and eventually secondary multiorgan failure due to right ventricular system failure were reported by Jakstaite et al. [16] in a 61-year-old male patient who had been using a continuous-flow LVAD (CF-LAVD) for 7 years.
Because of the different types of LVADs used, multiple study populations, follow-up times, and various etiologies of HF in patients, as well as the differences between results from single-center and multicenter trials, the reported incidence of VA after LVAD implantation is not identical in different medical centers. Harding et al. [17] and Andersen et al. [18] conducted single-center investigations with sample sizes of 17 and 23 patients, respectively, and observed a 59% and 52% incidence of postoperative VA, respectively. The difference between the two studies is the type of LVAD implanted, which was a pulsatile flow pump in the study by Harding and a continuous flow pump in the study by Andersen. Garan et al. [19] studied clinical data from 162 LVAD-assisted patients and discovered a 23.5% incidence of early postoperative VA (within 30 days of surgery). Cantillon et al. [20] reviewed the clinical data from 478 patients who underwent LVAD implantations at Cleveland Medical Center between 1991 and 2008 and found that the incidence of postoperative VA was 28.9%. Yap et al. [7] focused on 204 patients with LVAD implantations at two medical facilities in the Netherlands and found that 30.4% of them experienced postoperative VA. Miller et al. [10] conducted a multicenter study with 133 patients who had a continuous flow pump implanted and found that 24% of the patients developed postoperative VA. Bedi et al. [21] evaluated the clinical data from 111 patients who underwent LVAD implantations and discovered that 22% of them had postoperative VA.
Bedi et al. [21] discovered a 54% mortality risk for the development of VA within 1 week after LVAD implantation; patients with VA during LVAD support had a considerably higher mortality rate (33% vs. 18%, p < 0.001) than patients without VA [21]. Brenyo et al. [22] retrospectively reviewed clinical data from 61 patients with LVADs and reported that VA that developed within 1 month of LVAD implantation was related to a 45% mortality rate, and multivariate analysis revealed that postoperative VA was a predictor of higher mortality risk (HR = 9.69, p = 0.001). Galand et al. [43] showed that early VA after LVAD implantation was the greatest predictor of postoperative death within 30 days after the operation (OR = 7.36, p = 0.001).
Discussion
Overall, we discovered numerous significant tendencies in the process of VA after patients received LVAD. About 60% of patients who developed VA after LVAD implantation had a prior VA history, while nearly 40% had a new-onset VA. The majority of patients who occurred VA within the first 4 weeks of CF-LVAD implantation, and around half of them had symptomatic VA. When compared to historical controls, our research found that short-term mortality in patients with VA after LVAD implantation was equivalent to that of patients without VA, but long-term mortality was greatly higher.
Mechanisms of VA Occurrence
Autonomic nerve dysfunction, aberrant sympathetic activation, electrolyte imbalances, and other factors may lead to arrhythmias in patients with severe HF [24, 25]. Although earlier studies reported that LVAD assistance aids in the rehabilitation of cardiac autonomic nerve function in patients with severe HF [26], preoperative dysfunction might still be a source of postoperative VA. At present, the mechanism of VA after LVAD implantation is summarized as primary cardiomyopathy-related, device mechanical stimulation, myocardial scarring, ventricular displacement, electrolyte regulation, and other mechanisms [8].
Mechanical Effect
According to the most recent INTERMACS report, CF-LAVD is gradually replacing pulsatile blood flow pumps and has become the first choice for clinical application [27]. Compared to a pulsatile blood flow pump, CF-LAVD is less dependent on preload; however, when preload is significantly reduced or pump rpm is increased, the negative pressure of the inflow tract may aspirate the ventricular septum or the left ventricular free wall into the device, resulting in a “suction event” that leads to myocardial mechanical stimulation and VA [5]. According to Vollkron et al. [28], VA related to a “suction event” occurs suddenly and is relieved with the recovery of preload or the decrease in pump speed.
Furthermore, mechanical stimulation of the device may result in alterations in cardiac structure, changing electrical properties, and aberrant cardiomyocyte repolarization [4]. A prolonged QTc interval usually indicates aberrant cardiomyocyte repolarization. On the first day after LVAD implantation, a longer QTc interval is related to a higher incidence of postoperative VA (RR = 3.3, p = 0.01) [17]. The QTc interval increased from 479 at 10 ms to 504 at 11 ms in the first week after LVAD implantation and then decreased to 445 at 11 ms in the second week, according to Harding et al. [29]. Summarizing the results of these two studies, the QTc interval gradually decreases with the extension of LVAD support time, which may explain why VA usually occurs in the early stage after LVAD implantation.
Scar-Related Reentrant Arrhythmia
Potential cardiomyopathy after LVAD implantation is frequently accompanied by visible scar formation, which may be the cause of reentrant arrhythmia [30]. A meta-analysis showed that scar-related reentrant arrhythmia is the main mechanism of VT after LVAD implantation [31]. Sutures used in surgical procedures can cause myocardial fibrosis [32]. Pathological studies have shown that stable muscle fibrosis increases the likelihood of arrhythmia by altering cardiomyocyte excitability and increasing ectopic activity [33]. A pathological analysis of the LVAD inflow tract in the myocardium revealed that myocardial fibrosis can progress to apical scar formation, causing VA [34]. Simultaneously, the LVAD inflow channel that penetrates the left ventricular wall forms a special barrier that potentially causes reentrant arrhythmia [9].
Electrolyte Regulation
Regulation of the myocardial electrolyte concentration is critical for preserving proper cardiac function. Patients with chronic HF frequently have electrolyte disorders due to changes in neurohumoral regulation and self-regulation as well as the use of diuretic drugs (such as furosemide) and potassium supplement drugs (such as potassium chloride) [35]. However, as tissue and organ perfusion increase following LVAD implantation, various body regulatory systems change, resulting in electrolyte transfer. Ziv et al. [9] retrospectively analyzed the preoperative and postoperative clinical data from 100 patients with LVAD. A multivariate Cox regression analysis showed that abnormal serum electrolyte levels were an independent risk factor for postoperative VA. Monreal and Gerhardt [36] suggested that rapid changes in the electrolyte balance after LVAD implantation increase the risk of postoperative arrhythmias.
Others
VA is associated with delayed depolarization of cardiomyocytes, an increase in sodium-calcium exchange, and a change in the calcium concentration [37, 38]. Terracciano et al. [37] discovered that LVAD implantation improves normal calcium homeostasis and reduces abnormal calcium channel regulation in the myocardium of individuals with HF. In addition to the previously mentioned reasons, LVAD implantation may alter the expression of various genes involved in arrhythmia, such as upregulating actin and Ca2+ regulatory genes and downregulating connexin 43 and Na+/K+ ATPase expression [8]. Simultaneously, inotropic drugs used soon after LVAD are proposed to be related to early VA [8].
Risk Predictors
The risk predictors of VA following LVAD implantation vary depending on the type of LVAD and respondents of the study, similar to epidemiological studies. An understanding of the risk factors or predictors of VA may help doctors diagnose the disease early and intervene when necessary [39]. A preoperative VA history is considered a predictor of VA following LVAD implantation in most studies and, in some cases, is the only predictor of long-term VA after LVAD implantation [19, 23, 40, 41, 42, 43, 44]. Raasch et al. [45] studied 61 LVAD-assisted patients. Only a history of VA prior to implantation was found to be related to the occurrence of VA after LVAD (OR = 13.7, p = 0.001). Garan et al. [19] showed that a preoperative VA history (OR = 2.76, p = 0.02) and age (OR = 1.04, p = 0.03) were predictors of early postoperative VA (within 30 days after operation). Hellman et al. [46] performed a multivariate logistic regression analysis in a retrospective study with a sample size of 85 patients and revealed that a preoperative VA history was not a predictor, and only type B natriuretic peptide level (95% CI: of OR = 1.5–5.1, p = 0.0008) was a predictor of postoperative VA in this sample.
Galand et al. [42] collected clinical data from 659 LVAD-assisted patients in 19 medical facilities. Preoperative VA history (OR = 2.32, p = 0.001), preoperative atrial fibrillation history (OR = 1.72, p = 0.009), idiopathic cardiomyopathy (OR = 1.50, p = 0.045), HF duration (>12 months) (OR = 2.58, p = 0.001), and no use of angiotensin converting enzyme inhibitors (OR = 2.14, p = 0.001) were all found to be risk factors for postoperative VA in the multivariate analysis. Martins et al. [23] also conducted a 652-case multicenter study. In addition to a preoperative VA history (HR = 2.62, p = 0.001), the duration of HF (>84 months) (HR = 1.97, p = 0.028) was a predictor of postoperative VA.
In addition to the aforementioned risk factors and predictors, the genesis and postoperative management of cardiomyopathy have steadily attracted attention. Ziv et al. [9] and Bedi et al. [21] argue that patients with ischemic cardiomyopathy are more susceptible to developing VA following surgery. Nonischemic cardiomyopathy was reported to be a predictor of early postoperative VA (OR = 2.47, p = 0.046) by Garan et al. [19]. However, according to Corre et al. [66], the occurrence of VA following LVAD implantation is unrelated to the type of cardiomyopathy. Refaat et al. [48] analyzed patients with LVAD as the research object in addition to the above risk factors and predictors. VA and nonuse after LVAD implantation were related to the use of receptor blockers (OR = 7.04, p = 0.001) [48]. However, a single-center investigation with a sample size of 23 patients found that a lack of use of β-receptor blockers following surgery is not linked to arrhythmia [18].
Surveillance and Treatment
Multiple mechanisms and factors should be considered for the prevention and treatment of postoperative VA in patients with long-term VAD assistance. Pharmacotherapy is a key link in the management of postoperative VA according to the causes and risk factors discussed in the preceding sections, in addition to the preventative and therapeutic measures described above. β-Receptor blockers have been shown to exert a strong antiarrhythmic effect [49]. Although information on VAD from randomized and prospective studies on the use of receptor blockers is currently lacking, receptor blockers may be used in patients without significant contraindications, such as right ventricular dysfunction, based on a comprehensive consideration of individualized therapy. In addition to using routine medication (e.g., receptor blockers, amiodarone, lidocaine, etc.), clinicians should pay close attention to the changes in electrolyte levels, correct electrolyte disorders, and comprehensively analyze changes in hemodynamics and the causes of VA. Effective monitoring or etiological treatment should be implemented.
Prevention of “Suction Events”
“Suction events” have been identified as a common cause of VA following LVAD implantation. During monitoring and treatment, “suction events” are frequently characterized by a reduced flow, a characteristic flow profile, and increased power of the blood pump operation, among others, and should be noted and corrected as soon as possible. Body position may affect the spatial positional relationship of the inflow tract with the interventricular septum and ventricular free wall. Thus, real-time assessments of left ventricular geometry using perioperative echocardiography are also critical to avoid postoperative VA due to mechanical stimulation [50]. Adjusting the pump speed once a “suction event” occurs helps to restore the left ventricular volume status and reduces the likelihood of VA occurrence [51]. If changes in pump speed or volume status are insufficient to correct VA caused by contact between the VAD inflow tract and the myocardium, reoperation with adjustments to the access tract position should be considered [5].
Ablation
After a thorough assessment of the patient's situation, treatments such as ablation should be performed before VAD implantation in patients with a history of preoperative VA to increase the patient's survival rate after VAD implantation [52]. If the patient's preoperative conditions do not allow ablation prior to implantation, surgical ablation during the same period of VAD implantation should be considered [53]. VA ablation will be limited after LVAD implantation. On the one hand, a pericardial adhesion is generated after VAD implantation, essentially resulting in a limitation on epicardial ablation; on the other hand, the VAD cannula limits the use of intracardiac ablation. However, for patients with medically uncontrolled VA after LVAD implantation, intracardiac ablation remains an option. Among a sample size of 34 patients who experienced VA following HeartMate II implantation, 13 were free of recurrent arrhythmias after 25 ± 15 months of follow-up [51]. Another study involved 21 patients (15 HeartMate II®, 6 HeartWare®) and found that 64% of patients were free of recurrent VA after ablation, with 1-year survival rates of 67% for nonrecurrent patients and 29% for recurrent patients (p = 0.049) [54].
Implanted Cardioverter Defibrillators
Implantable cardioverter defibrillators (ICDs) are currently considered primary and secondary prevention techniques for sudden cardiac death in patients with HF, but perspectives on their utility in patients following VAD implantation are mixed [55]. A case of electromagnetic interference produced by ICD and LVAD was described by Moini et al. [56]. Erqou et al. [47] described a case in which the LVAD interfered with the ICD function due to electromagnetic interference. According to previous studies, an interaction between LVAD and ICD devices is uncommon, with only 2 of 76 patients (HeartMate II®) experiencing such an interaction [57]. Yalcin et al. [58] performed a single-center retrospective study to analyze the clinical data from 86 patients with LVAD and ICD implantation (46 patients implanted with HeartMate II® and 40 patients implanted with HeartMate III®). The incidence of electromagnetic interference in patients implanted with the HeartMate II® and ICD or HeartMate III® and ICD was 15% and 11%, respectively.
Others
By decreasing sympathetic activity, sympathetic denervation has been shown to diminish the occurrence of persistent VA or the usage of ICD [59]. Vlismas et al. [60] reported that sympathetic denervation was performed in 1 female patient with intermittent VA after LVAD implantation, and no recurrence was observed at the 8-month postoperative follow-up, implying that sympathetic denervation may be considered for patients with postoperative VA implanted with LVADs who are refractory to medical, ablative, or ICD therapy.
Conclusion and Outlook
With the widespread use of LVAD in clinical practice, postoperative VA has become a common clinical complication. Although postoperative VA with LVAD has no effect on short-term hemodynamics in many individual patients, its long-term persistence or recurrence still poses risks of increased postoperative mortality and rehospitalization. By summarizing case data from various centers, we discovered that even if severe VA (such as VF) occurs, patients still have the possibility of maintaining hemodynamic stability with the support of LVAD; however, the ability to maintain circulation remains unclear. Our study revealed that before LVAD is implanted, the burden of arrhythmias should be considered and thoroughly assessed because the prior VA is the strongest predictor for post-LVAD VA. Even though post-LVAD VA occurs commonly in post-LVAD patients, it did not significantly increase the mortality. Ablation has shown promise as a routine strategy to prevent or cure postoperative VA by preventing “suction events.” The therapeutic efficacy and long-term effects of ICD and sympathetic denervation are not fully understood.
In the future, relevant acute and chronic animal experiments should be conducted to examine the effect of the postoperative VA duration on vital signs and circulatory conditions, as well as the reasons for short-term hemodynamic stability during VF. New management strategies that contain patient baseline features, prior history of VA, proper scheduling of ablation therapy, and effective medical treatment will certainly help us better understand VA occurrence after LVAD implantation and the final endings in LVAD patients. Future multicenter prospective studies with uniform patient follow-up methods are needed to screen additional potential risk factors and predictors. This approach will help define the incidence rate of VA after LAVD implantation. As a result, we will be able to guide the selection of preventive interventions.
Limitations
The majority of current relevant studies are single-center, small-sample, and retrospective, and the risk factors and predictors examined may be insufficient and vary widely and additional multicenter prospective studies are needed. Another limitation is the inclusion of studies that examined CF-LVAD patients who specifically received ablation for the treatment of VA. This may have raised the rate of ablation in our population inadvertently.
Statement of Ethics
An ethics statement was not required for this study type, no human or animal subjects or materials were used.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was supported by grants from the National Key Research and Development Program of China (Project number: 2017YFC0111005) and Natural Science Foundation of Tianjin, China (Project number: 18JCZDJC36200).
Author Contributions
Jianwei Shi: conception/design, design, data collection, and drafting the article. Xinyi Yu: data collection and data interpretation. Zhigang Liu: conception/design, data interpretation, critical revision of the article, and approval of the article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
References
- 1.Writing C, Maddox TM, Januzzi JL, Jr, Allen LA, Breathett K, Butler J, et al. 2021 update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction − a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021 Feb 16;77((6)):772–810. doi: 10.1016/j.jacc.2020.11.022. [DOI] [PubMed] [Google Scholar]
- 2.Hao G, Wang X, Chen Z, Zhang L, Zhang Y, Wei B, et al. Prevalence of heart failure and left ventricular dysfunction in China: the China Hypertension Survey, 2012–2015. Eur J Heart Fail. 2019 Nov;21((11)):1329–37. doi: 10.1002/ejhf.1629. [DOI] [PubMed] [Google Scholar]
- 3.Molina EJ, Shah P, Kiernan MS, Cornwell WK, 3rd, Copeland H, Takeda K, et al. The society of thoracic surgeons intermacs 2020 annual report. Ann Thorac Surg. 2021 Mar;111((3)):778–92. doi: 10.1016/j.athoracsur.2020.12.038. [DOI] [PubMed] [Google Scholar]
- 4.Nakahara S, Chien C, Gelow J, Dalouk K, Henrikson CA, Mudd J, et al. Ventricular arrhythmias after left ventricular assist device. Circ Arrhythm Electrophysiol. 2013 Jun;6((3)):648–54. doi: 10.1161/CIRCEP.113.000113. [DOI] [PubMed] [Google Scholar]
- 5.Gopinathannair R, Cornwell WK, Dukes JW, Ellis CR, Hickey KT, Joglar JA, et al. Device therapy and arrhythmia management in left ventricular assist device recipients: a scientific statement from the American Heart Association. Circulation. 2019 May 14;139((20)):e967–89. doi: 10.1161/CIR.0000000000000673. [DOI] [PubMed] [Google Scholar]
- 6.Griffin JM, Katz JN. The burden of ventricular arrhythmias following left ventricular assist device implantation. Arrhythm Electrophysiol Rev. 2014 Nov;3((3)):145–8. doi: 10.15420/aer.2014.3.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yap SC, Ramjankhan F, Muslem R, de Jonge N, Kirkels HJ, Akin S, et al. Ventricular arrhythmias in patients with a continuous-flow left ventricular assist device. J Am Coll Cardiol. 2016 Jul 19;68((3)):323–5. doi: 10.1016/j.jacc.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 8.Kadado AJ, Akar JG, Hummel JP. Arrhythmias after left ventricular assist device implantation: incidence and management. Trends Cardiovasc Med. 2018 Jan;28((1)):41–50. doi: 10.1016/j.tcm.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Ziv O, Dizon J, Thosani A, Naka Y, Magnano AR, Garan H. Effects of left ventricular assist device therapy on ventricular arrhythmias. J Am Coll Cardiol. 2005 May 3;45((9)):1428–34. doi: 10.1016/j.jacc.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 10.Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007 Aug 30;357((9)):885–96. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 11.Bouchez S, De Somer F, Herck I, Van Belleghem Y, De Pauw M, Stroobandt R. Shock-refractory ventricular fibrillation in a patient implanted with a left ventricular assist device. Resuscitation. 2016 Oct;107:e1–2. doi: 10.1016/j.resuscitation.2016.06.034. [DOI] [PubMed] [Google Scholar]
- 12.Smith ME, Moak JH. Asymptomatic ventricular fibrillation in continuous flow left-ventricular assist device. Am J Emerg Med. 2021 Nov;49:130–2. doi: 10.1016/j.ajem.2021.05.065. [DOI] [PubMed] [Google Scholar]
- 13.Busch MC, Haap M, Kristen A, Haas CS. Asymptomatic sustained ventricular fibrillation in a patient with left ventricular assist device. Ann Emerg Med. 2011 Jan;57((1)):25–8. doi: 10.1016/j.annemergmed.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 14.Patel P, Williams JG, Brice JH. Sustained ventricular fibrillation in an alert patient: preserved hemodynamics with a left ventricular assist device. Prehosp Emerg Care. 2011 Oct-Dec;15((4)):533–6. doi: 10.3109/10903127.2011.598616. [DOI] [PubMed] [Google Scholar]
- 15.Eyituoyo HO, Aben RN, Arinze NC, Vu DP, James EA. Ventricular fibrillation 7 years after left ventricular assist device implantation. Am J Case Rep. 2020 Jun 20;21:e923711. doi: 10.12659/AJCR.923711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jakstaite AM, Luedike P, Wakili R, Kochhauser S, Ruhparwar A, Rassaf T, et al. Case report: incessant ventricular fibrillation in a conscious left ventricular assist device patient. Eur Heart J Case Rep. 2021 Sep;5((9)):ytab337. doi: 10.1093/ehjcr/ytab337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harding JD, Piacentino V, 3rd, Rothman S, Chambers S, Jessup M, Margulies KB. Prolonged repolarization after ventricular assist device support is associated with arrhythmias in humans with congestive heart failure. J Card Fail. 2005 Apr;11((3)):227–32. doi: 10.1016/j.cardfail.2004.08.158. [DOI] [PubMed] [Google Scholar]
- 18.Andersen M, Videbaek R, Boesgaard S, Sander K, Hansen PB, Gustafsson F. Incidence of ventricular arrhythmias in patients on long-term support with a continuous-flow assist device (HeartMate II) J Heart Lung Transplant. 2009 Jul;28((7)):733–5. doi: 10.1016/j.healun.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Garan AR, Levin AP, Topkara V, Thomas SS, Yuzefpolskaya M, Colombo PC, et al. Early post-operative ventricular arrhythmias in patients with continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2015 Dec;34((12)):1611–6. doi: 10.1016/j.healun.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Cantillon DJ, Tarakji KG, Kumbhani DJ, Smedira NG, Starling RC, Wilkoff BL. Improved survival among ventricular assist device recipients with a concomitant implantable cardioverter-defibrillator. Heart Rhythm. 2010 Apr;7((4)):466–71. doi: 10.1016/j.hrthm.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 21.Bedi M, Kormos R, Winowich S, McNamara DM, Mathier MA, Murali S. Ventricular arrhythmias during left ventricular assist device support. Am J Cardiol. 2007 Apr 15;99((8)):1151–3. doi: 10.1016/j.amjcard.2006.11.051. [DOI] [PubMed] [Google Scholar]
- 22.Brenyo A, Rao M, Koneru S, Hallinan W, Shah S, Massey HT, et al. Risk of mortality for ventricular arrhythmia in ambulatory LVAD patients. J Cardiovasc Electrophysiol. 2012 May;23((5)):515–20. doi: 10.1111/j.1540-8167.2011.02223.x. [DOI] [PubMed] [Google Scholar]
- 23.Martins RP, Leclercq C, Bourenane H, Auffret V, Boule S, Loobuyck V, et al. Incidence, predictors, and clinical impact of electrical storm in patients with left ventricular assist devices: new insights from the ASSIST-ICD study. Heart Rhythm. 2019 Oct;16((10)):1506–12. doi: 10.1016/j.hrthm.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 24.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009 Nov 3;54((19)):1747–62. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 25.Kishi T. Heart failure as an autonomic nervous system dysfunction. J Cardiol. 2012 Mar;59((2)):117–22. doi: 10.1016/j.jjcc.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Kim SY, Montoya A, Zbilut JP, Mawulawde K, Sullivan HJ, Lonchyna VA, et al. Effect of HeartMate left ventricular assist device on cardiac autonomic nervous activity. Ann Thorac Surg. 1996 Feb;61((2)):591–3. doi: 10.1016/0003-4975(95)01086-6. [DOI] [PubMed] [Google Scholar]
- 27.Teuteberg JJ, Cleveland JC, Jr, Cowger J, Higgins RS, Goldstein DJ, Keebler M, et al. The society of thoracic surgeons intermacs 2019 annual report: the changing landscape of devices and indications. Ann Thorac Surg. 2020 Mar;109((3)):649–60. doi: 10.1016/j.athoracsur.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Vollkron M, Voitl P, Ta J, Wieselthaler G, Schima H. Suction events during left ventricular support and ventricular arrhythmias. J Heart Lung Transplant. 2007 Aug;26((8)):819–25. doi: 10.1016/j.healun.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Harding JD, Piacentino V, 3rd, Gaughan JP, Houser SR, Margulies KB. Electrophysiological alterations after mechanical circulatory support in patients with advanced cardiac failure. Circulation. 2001 Sep 11;104((11)):1241–7. doi: 10.1161/hc3601.095718. [DOI] [PubMed] [Google Scholar]
- 30.Maradey JA, Singleton MJ, O'Neill TJ, Bhave PD. Management of ventricular arrhythmias in patients with LVAD. Curr Opin Cardiol. 2020 May;35((3)):289–94. doi: 10.1097/HCO.0000000000000730. [DOI] [PubMed] [Google Scholar]
- 31.Anderson RD, Lee G, Virk S, Bennett RG, Hayward CS, Muthiah K, et al. Catheter ablation of ventricular tachycardia in patients with a ventricular assist device: a systematic review of procedural characteristics and outcomes. JACC Clin Electrophysiol. 2019 Jan;5((1)):39–51. doi: 10.1016/j.jacep.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Arkles JS, Marchlinski F. When should the electrophysiologist be involved in managing patients with ventricular assist devices and ventricular arrhythmias? J Innov Card Rhythm Manag. 2019 Apr;10((4)):3605–10. doi: 10.19102/icrm.2019.100407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Askar SF, Ramkisoensing AA, Schalij MJ, Bingen BO, Swildens J, van der Laarse A, et al. Antiproliferative treatment of myofibroblasts prevents arrhythmias in vitro by limiting myofibroblast-induced depolarization. Cardiovasc Res. 2011 May 1;90((2)):295–304. doi: 10.1093/cvr/cvr011. [DOI] [PubMed] [Google Scholar]
- 34.Strecker T, Rösch J, Weyand M, Agaimy A. Pathological findings in cardiac apex removed during implantation of left ventricular assist devices (LVAD) are non-specific: 13-year-experience at a German Heart Center. Int J Clin Exp Pathol. 2014;7((9)):5549–56. [PMC free article] [PubMed] [Google Scholar]
- 35.Urso C, Brucculeri S, Caimi G. Acid-base and electrolyte abnormalities in heart failure: pathophysiology and implications. Heart Fail Rev. 2015 Jul;20((4)):493–503. doi: 10.1007/s10741-015-9482-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monreal G, Gerhardt MA. Left ventricular assist device support induces acute changes in myocardial electrolytes in heart failure. ASAIO J. 2007 Mar–Apr;53((2)):152–8. doi: 10.1097/MAT.0b013e3180302a8b. [DOI] [PubMed] [Google Scholar]
- 37.Terracciano CM, Harding SE, Adamson D, Koban M, Tansley P, Birks EJ, et al. Changes in sarcolemmal Ca entry and sarcoplasmic reticulum Ca content in ventricular myocytes from patients with end-stage heart failure following myocardial recovery after combined pharmacological and ventricular assist device therapy. Eur Heart J. 2003 Jul;24((14)):1329–39. doi: 10.1016/s0195-668x(03)00242-2. [DOI] [PubMed] [Google Scholar]
- 38.Tomaselli GF. Calcium and arrhythmias: ignore at your peril. J Cardiovasc Electrophysiol. 2012 Dec;23((12)):1372–3. doi: 10.1111/j.1540-8167.2012.02423.x. [DOI] [PubMed] [Google Scholar]
- 39.Mulloy DP, Bhamidipati CM, Stone ML, Ailawadi G, Bergin JD, Mahapatra S, et al. Cryoablation during left ventricular assist device implantation reduces postoperative ventricular tachyarrhythmias. J Thorac Cardiovasc Surg. 2013 May;145((5)):1207–13. doi: 10.1016/j.jtcvs.2012.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oswald H, Schultz-Wildelau C, Gardiwal A, Lusebrink U, Konig T, Meyer A, et al. Implantable defibrillator therapy for ventricular tachyarrhythmia in left ventricular assist device patients. Eur J Heart Fail. 2010 Jun;12((6)):593–9. doi: 10.1093/eurjhf/hfq048. [DOI] [PubMed] [Google Scholar]
- 41.Garan AR, Yuzefpolskaya M, Colombo PC, Morrow JP, Te-Frey R, Dano D, et al. Ventricular arrhythmias and implantable cardioverter-defibrillator therapy in patients with continuous-flow left ventricular assist devices: need for primary prevention? J Am Coll Cardiol. 2013 Jun 25;61((25)):2542–50. doi: 10.1016/j.jacc.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 42.Galand V, Flecher E, Auffret V, Boule S, Vincentelli A, Dambrin C, et al. Predictors and clinical impact of late ventricular arrhythmias in patients with continuous-flow left ventricular assist devices. JACC Clin Electrophysiol. 2018 Sep;4((9)):1166–75. doi: 10.1016/j.jacep.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 43.Galand V, Flecher E, Auffret V, Pichard C, Boule S, Vincentelli A, et al. Early ventricular arrhythmias after LVAD implantation is the strongest predictor of 30-day post-operative mortality. JACC Clin Electrophysiol. 2019 Aug;5((8)):944–54. doi: 10.1016/j.jacep.2019.05.025. [DOI] [PubMed] [Google Scholar]
- 44.Rehorn MR, Black-Maier E, Loungani R, Sen S, Sun AY, Friedman DJ, et al. Electrical storm in patients with left ventricular assist devices: risk factors, incidence, and impact on survival. Heart Rhythm. 2021 Aug;18((8)):1263–71. doi: 10.1016/j.hrthm.2021.03.047. [DOI] [PubMed] [Google Scholar]
- 45.Raasch H, Jensen BC, Chang PP, Mounsey JP, Gehi AK, Chung EH, et al. Epidemiology, management, and outcomes of sustained ventricular arrhythmias after continuous-flow left ventricular assist device implantation. Am Heart J. 2012 Sep;164((3)):373–8. doi: 10.1016/j.ahj.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 46.Hellman Y, Malik AS, Lin H, Shen C, Wang IW, Wozniak TC, et al. B-type natriuretic peptide levels predict ventricular arrhythmia post left ventricular assist device implantation. Artif Organs. 2015 Dec;39((12)):1051–5. doi: 10.1111/aor.12486. [DOI] [PubMed] [Google Scholar]
- 47.Erqou S, Kormos RL, Wang NC, McNamara DM, Bazaz R. Electromagnetic interference from left ventricular assist device (LVAD) inhibiting the pacing function of an implantable cardioverter-defibrillator (ICD) device. Case Rep Cardiol. 2018;2018:6195045. doi: 10.1155/2018/6195045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Refaat M, Chemaly E, Lebeche D, Gwathmey JK, Hajjar RJ. Ventricular arrhythmias after left ventricular assist device implantation. Pacing Clin Electrophysiol. 2008 Oct;31((10)):1246–52. doi: 10.1111/j.1540-8159.2008.01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grandi E, Ripplinger CM. Antiarrhythmic mechanisms of beta blocker therapy. Pharmacol Res. 2019 Aug;146:104274. doi: 10.1016/j.phrs.2019.104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stainback RF, Estep JD, Agler DA, Birks EJ, Bremer M, Hung J, et al. Echocardiography in the management of patients with left ventricular assist devices: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2015 Aug;28((8)):853–909. doi: 10.1016/j.echo.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 51.Sacher F, Reichlin T, Zado ES, Field ME, Viles-Gonzalez JF, Peichl P, et al. Characteristics of ventricular tachycardia ablation in patients with continuous flow left ventricular assist devices. Circ Arrhythm Electrophysiol. 2015 Jun;8((3)):592–7. doi: 10.1161/CIRCEP.114.002394. [DOI] [PubMed] [Google Scholar]
- 52.Pedrotty DM, Rame JE, Margulies KB. Management of ventricular arrhythmias in patients with ventricular assist devices. Curr Opin Cardiol. 2013 May;28((3)):360–8. doi: 10.1097/HCO.0b013e32835fb7dc. [DOI] [PubMed] [Google Scholar]
- 53.Emaminia A, Nagji AS, Ailawadi G, Bergin JD, Kern JA. Concomitant left ventricular assist device placement and cryoablation for treatment of ventricular tachyarrhythmias associated with heart failure. Ann Thorac Surg. 2011 Jul;92((1)):334–6. doi: 10.1016/j.athoracsur.2010.12.062. [DOI] [PubMed] [Google Scholar]
- 54.Moss JD, Flatley EE, Beaser AD, Shin JH, Nayak HM, Upadhyay GA, et al. Characterization of ventricular tachycardia after left ventricular assist device implantation as destination therapy: a single-center ablation experience. JACC Clin Electrophysiol. 2017 Dec 11;3((12)):1412–24. doi: 10.1016/j.jacep.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 55.Refaat MM, Tanaka T, Kormos RL, McNamara D, Teuteberg J, Winowich S, et al. Survival benefit of implantable cardioverter-defibrillators in left ventricular assist device-supported heart failure patients. J Card Fail. 2012 Feb;18((2)):140–5. doi: 10.1016/j.cardfail.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moini C, Lefoulon A, Rahim D, Yassine M, Poindron D, Amara W. [ICD and left ventricular assist device interference: case report] Ann Cardiol Angeiol. 2020 Nov;69((5)):332–4. doi: 10.1016/j.ancard.2020.09.029. [DOI] [PubMed] [Google Scholar]
- 57.Kuhne M, Sakumura M, Reich SS, Sarrazin JF, Wells D, Chalfoun N, et al. Simultaneous use of implantable cardioverter-defibrillators and left ventricular assist devices in patients with severe heart failure. Am J Cardiol. 2010 Feb 1;105((3)):378–82. doi: 10.1016/j.amjcard.2009.09.044. [DOI] [PubMed] [Google Scholar]
- 58.Yalcin YC, Kooij C, Theuns D, Constantinescu AA, Brugts JJ, Manintveld OC, et al. Emerging electromagnetic interferences between implantable cardioverter-defibrillators and left ventricular assist devices. Europace. 2020 Apr 1;22((4)):584–7. doi: 10.1093/europace/euaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vaseghi M, Barwad P, Malavassi Corrales FJ, Tandri H, Mathuria N, Shah R, et al. Cardiac sympathetic denervation for refractory ventricular arrhythmias. J Am Coll Cardiol. 2017 Jun 27;69((25)):3070–80. doi: 10.1016/j.jacc.2017.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vlismas PP, Rochlani YM, Romero J, Scheinin S, Shin JJ, Goldstein D, et al. Cardiac sympathetic denervation for refractory ventricular arrhythmia in continuous-flow left ventricular assist device. JACC Case Rep. 2021 Mar;3((3)):443–6. doi: 10.1016/j.jaccas.2020.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boudghène-Stambouli F, Boulé S, Goéminne C, Botcherby E, Marquié C, Kouakam C, et al. Clinical implications of left ventricular assist device implantation in patients with an implantable cardioverter-defibrillator. J Interv Card Electrophysiol. 2013;39((2)):177–84. doi: 10.1007/s10840-013-9854-y. [DOI] [PubMed] [Google Scholar]
- 62.Efimova E, Fischer J, Bertagnolli L, Dinov B, Kircher S, Rolf S, et al. Predictors of ventricular arrhythmia after left ventricular assist device implantation: A large single-center observational study. Heart Rhythm. 2017;14((12)):1812–19. doi: 10.1016/j.hrthm.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 63.Enriquez AD, Calenda B, Miller MA, Anyanwu AC, Pinney SP. The Role of Implantable Cardioverter-Defibrillators in Patients With Continuous Flow Left Ventricular Assist Devices. Circ Arrhythm Electrophysiol. 2013;6((4)):668–74. doi: 10.1161/CIRCEP.113.000457. [DOI] [PubMed] [Google Scholar]
- 64.Yoruk A, Sherazi S, Massey HT, Kutyifa V, McNitt S, Hallinan W, et al. Predictors and clinical relevance of ventricular tachyarrhythmias in ambulatory patients with a continuous flow left ventricular assist device. Heart Rhythm. 2016;13((5)):1052–56. doi: 10.1016/j.hrthm.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 65.Kumar A, Tandon V, O'Sullivan DM, Cronin E, Gluck J, Kluger J. ICD shocks in LVAD patients are not associated with increased subsequent mortality risk. J Interv Card Electrophysiol. 2019;56((3)):341–48. doi: 10.1007/s10840-019-00619-7. [DOI] [PubMed] [Google Scholar]
- 66.Corre J, Picard F, Garcia R, Zemmoura A, Derval N, Denis A, et al. Electrical storm in the early phase of HeartMate® II device implantation: Incidence, risk factors and prognosis. Arch Cardiovasc Dis. 2018;111((5)):332–9. doi: 10.1016/j.acvd.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 67.Greet BD, Pujara D, Burkland D, Pollet M, Sudhakar D, Rojas F, et al. Incidence, Predictors, and Significance of Ventricular Arrhythmias in Patients With Continuous-Flow Left Ventricular Assist Devices: A 15-Year Institutional Experience. JACC Clin Electrophysiol. 2018;4((2)):257–64. doi: 10.1016/j.jacep.2017.11.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.