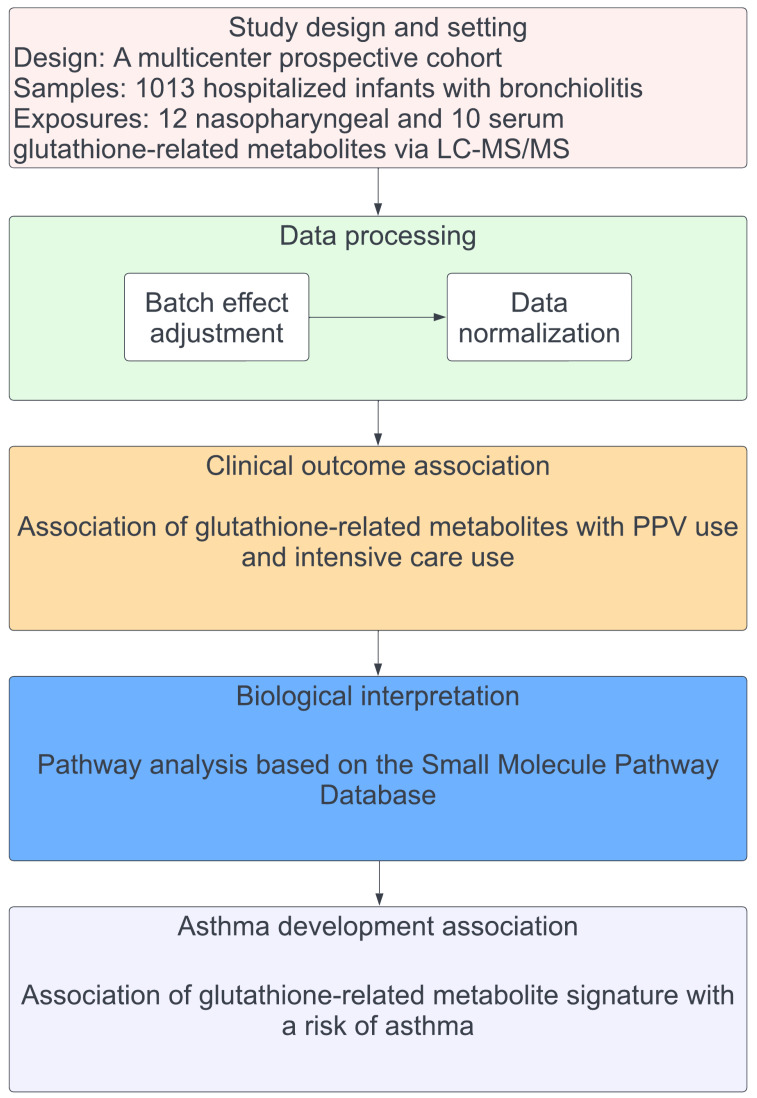
Figure 1.
Analytic workflow. The analytic cohort consisted of 1013 infants hospitalized for bronchiolitis in a multicenter prospective cohort study—MARC-35. In this cohort, 12 nasopharyngeal airway and 10 serum glutathione-related metabolites were identified by using liquid chromatography–tandem mass spectrometry (LC-MS/MS). The glutathione-related metabolites data were adjusted for the potential batch effect and normalized. The association of the glutathione-related metabolites with the risk of PPV use and intensive care use was estimated. To examine the biological function of the measured metabolites, metabolite set enrichment analysis was conducted. The nasopharyngeal and serum glutathione-related metabolite signatures for PPV use were estimated. Then, the association of the signature with the risk of developing asthma was estimated. Abbreviations: LC-MS/MS, liquid chromatography–tandem mass spectrometry; PPV, positive pressure ventilation.