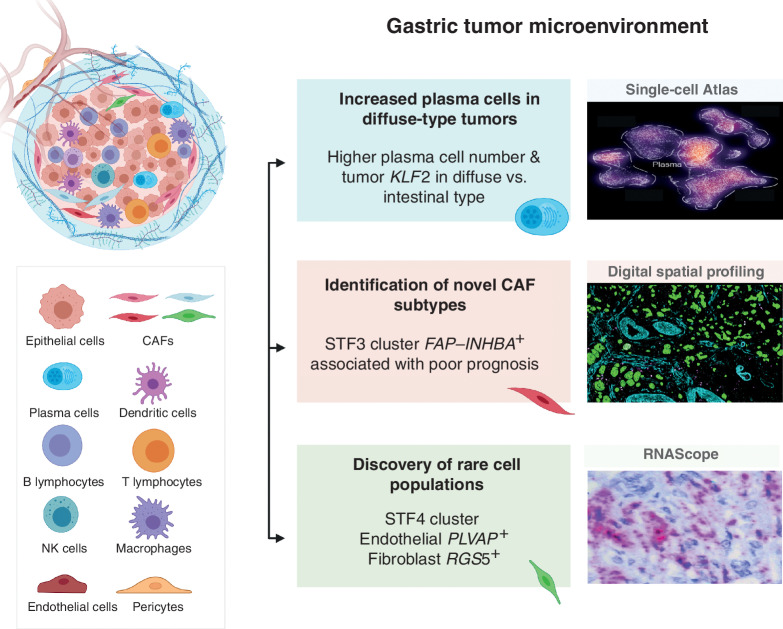
Figure 6.
Comprehensive single-cell atlas of gastric cancer. This study included more than 200,000 cells from 31 primary gastric tumor samples. In total, 34 distinct cell-lineage states were identified, related by developmental trajectories and previously unreported rare cell populations. An increase in plasma cell proportions was observed as a feature of diffuse-type tumors associated with epithelial-resident KLF2. A stage-wise accrual of novel cancer-associated fibroblast subpopulations was marked by high INHBA and FAP coexpression. Findings were complemented using digital spatial transcriptomics and RNAScope. Our results provide a high-resolution molecular resource for gastric cancer translational studies, identifying intra- and interpatient lineage states across distinct gastric cancer subtypes.