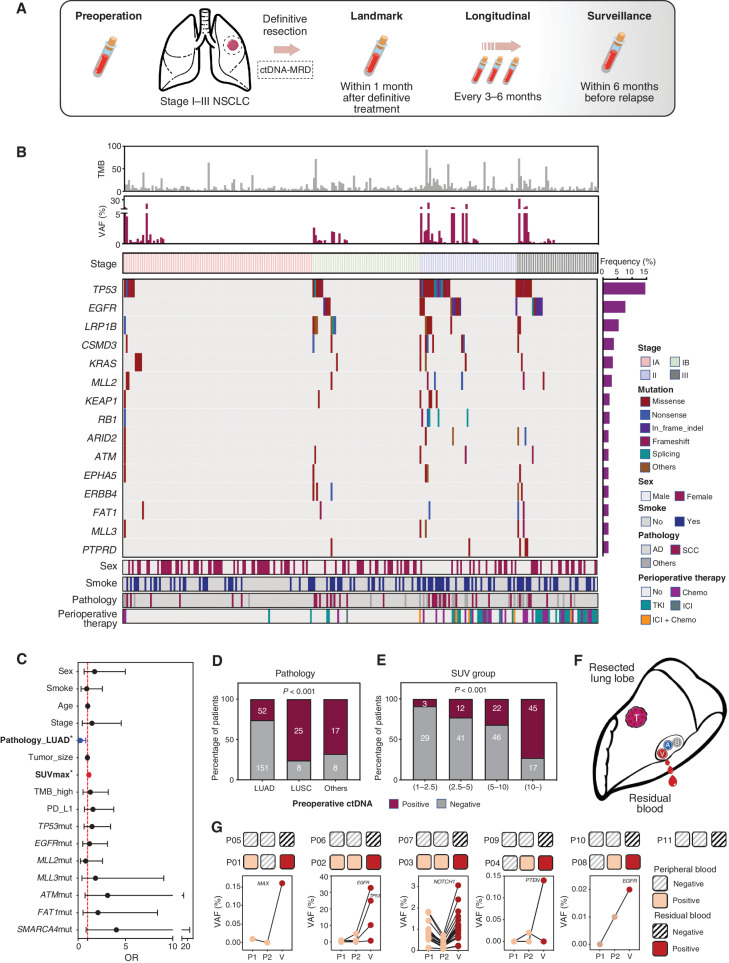
Figure 2.
Study schematic and baseline characteristics. A, Study flowchart. Patients with stage I to III NSCLC (tumor diameter ≥2 cm) treated with definitive surgery were enrolled. Peripheral blood samples were collected before surgery and every 3 to 6 months after surgery. B, Heat map plot based on baseline characteristics and preoperative ctDNA tests of each patient. C, Multivariate logistic regression model for preoperative ctDNA detection. Pathologic type (adenocarcinoma) and SUVmax were independently associated with preoperative ctDNA detection. D, Detection rate of preoperative ctDNA in patients with different pathologic types. E, Detection rate of preoperative ctDNA in patients with different groups of SUVmax subgroup. F, Schematic diagram of residual blood sample collection from resected lung lobe. G, Comparison between two peripheral blood samples and residual blood ctDNA analysis of additional 11 patients with stage I NSCLC. AD, adenocarcinoma; Chemo, chemotherapy; SCC, squamous cell carcinoma; TKI, tyrosine kinase inhibitor; TMB, tumor mutation burden.