Abstract
Background
Vascularized composite tissue allotransplantation (VCA) to replace limbs or faces damaged beyond repair is now possible. The resulting clear benefit to quality of life is a compelling reason to attempt this complex procedure. Unfortunately, the high doses of immunosuppressive drugs required to protect this type of allograft result in significant morbidity and mortality giving rise to ethical concerns about performing this surgery in patients with non‐life‐threatening conditions. Here we tested whether we could suppress anti‐graft immune activity by using a safe β2‐adrenergic receptor (AR) agonist, terbutaline, to mimic the natural immune suppression generated by nervous system‐induced signalling through AR.
Methods
A heterotopic hind limb transplantation model was used with C57BL/6 (H‐2b) as recipients and BALB/c (H‐2d) mice as donors. To test the modulation of the immune response, graft survival was investigated after daily intraperitoneal injection of β2‐AR agonist with and without tacrolimus. Analyses of immune compositions and quantification of pro‐inflammatory cytokines were performed to gauge functional immunomodulation. The contributions to allograft survival of β2‐AR signalling in donor and recipient tissue were investigated with β2‐AR−/− strains.
Results
Treatment with the β2‐AR agonist delayed VCA rejection, even with a subtherapeutic dose of tacrolimus. β2‐AR agonist decreased T‐cell infiltration into the transplanted grafts and decreased memory T‐cell populations in recipient's circulation. In addition, decreased levels of inflammatory cytokines (IFN‐γ, IL‐6, TNF‐α, CXCL‐1/10 and CCL3/4/5/7) were detected following β2‐AR agonist treatment, and there was a decreased expression of ICAM‐1 and vascular cell adhesion molecule‐1 in donor stromal cells.
Conclusions
β2‐AR agonist can be used safely to mimic the natural suppression of immune responses, which occurs during adrenergic stress‐signalling and thereby can be used in combination regimens to reduce the dose needed of toxic immunosuppressive drugs such as tacrolimus. This strategy can be further evaluated for feasibility in the clinic.
Keywords: immunosuppression, stress signalling, vascularized composite tissue allotransplantation, β2‐adrenergic receptors
Enhancing immunosuppression by adrenergic receptor manipulation using a β2‐agonist enhances VCA graft survival without increasing toxicity.

1. INTRODUCTION
Remarkable improvements in on‐site emergency care have led to an increased survival of patients with traumatic injuries that previously would have been fatal‐injuries which result from accidents, gun‐shots or explosions in both civilian and military settings. 1 , 2 , 3 However, these surviving patients must endure life‐long difficulties associated with the loss of legs, arms, hands or other functional units (including faces) with a significant decline in quality of life. This difficult situation has generated interest in testing new transplant protocols using body parts from cadaveric donors in a procedure known as vascularized composite tissue allotransplantation (VCA). With more than 100 patients receiving VCA over the past decade, this novel transplantation is raising hope for patients with devastating deformities and complex tissue defects. 4 , 5 , 6 , 7 , 8 In large part, these successes have been achieved both by improved microsurgical techniques (e.g. anastomoses of vessels and nerves) and the use of large doses of potent pharmacological agents to induce immunosuppression, including cyclosporine A, tacrolimus and mycophenolic acid. 9 , 10 , 11 , 12 However, despite the excitement surrounding VCA, significant challenges prevent its widespread acceptance and use. First and foremost is that tissues and organs recovered from cadaveric donors are a scare resource with little chance of a human leukocyte antigen (HLA)‐match with the recipient. 13 As a result, very heavy doses of immunosuppressive medications are required, exposing the patient to opportunistic infections, hyperglycaemia, hepatotoxicity, nephrotoxicity, cancer and reproductive toxicity. 8 , 14 , 15 Additionally, in vivo and in vitro studies suggest that the immunosuppressants cyclosporine and tacrolimus can promote carcinogenesis and cancer progression through production of transforming growth factor‐β, increasing tumour angiogenesis and metastasis. 16 , 17 Adverse reactions and toxicity often necessitate reduction, and even complete withdrawal, of immunosuppressive drugs leading to a tragic graft rejection and loss. As an example of this scenario, a patient who received the first face transplantation in 2005 suffered from two different types of cancer as a consequence of potent immunosuppression and subsequently lost her lips due to graft rejection. 18 Although VCA can provide significant improvement in quality of life, far too many of these patients experience either graft rejection or increased risk of additional health problems, including cancer, from the chronic use of high dose immunosuppressive drugs. 14 This has led to significant ethical concerns about using this type of transplant in patients when it is not medically ‘life‐saving’, unlike the situation for patients requiring solid organ transplants such as liver or heart, which are medically required to save their lives. Moreover, with solid organ transplants, there is usually an opportunity to plan ahead and achieve a donor HLA match. Overall, there is a serious and unmet medical need for new strategies to improve graft survival after VCA and lessen the risk of life‐threatening morbidities from toxic immunosuppressive drugs.
The nervous and immune systems have been found to interact closely in host defence and stress responses. 19 , 20 , 21 , 22 , 23 , 24 Although the relationship of the hypothalamus–pituitary–adrenal axis and cortisol has been well studied, 25 , 26 the natural role of the autonomic nervous system in regulating immune responses is receiving increased attention; sympathetic and parasympathetic nerves are found innervating immune organs and near immune cells throughout the body. Extensive research now shows that neurotransmitter interactions between norepinephrine (NE) and β‐adrenergic receptors (ARs) regulate the immune system. 27 , 28 , 29
Recently, we have shown that β2‐AR signalling has an important role in immune regulation of CD8+ T cells and myeloid‐derived suppressor cells (MDSC). 30 , 31 , 32 , 33 , 34 The strong, naturally occurring immunosuppressive potential of β‐AR signalling is consistent with our observations that adrenergic stress or addition of β‐AR agonists can suppress graft versus host disease (GVHD) following allogeneic bone marrow transplantation (BMT). 34 , 35 , 36
These data led us to investigate whether providing a pharmacological agonist of β‐AR, thus mimicking the natural neuro‐immune axis, could be exploited to suppress immune responses following VCA and permit a reduction in the dose of more toxic immunosuppressant drugs such as tacrolimus. Here, we investigated the impact of targeting β2‐AR, using the β2‐agonist terbutaline, on graft rejection rate and immune contexture using wild type (WT) and β2‐AR‐knock‐out (KO) mice. We found that increased β2‐AR signalling results in delayed rejection responses in VCA recipients without detectable toxicity and this occurred through mechanisms involving suppression of pro‐inflammatory cytokines and chemokine as well as inhibition of endothelial adhesion molecules need for infiltration of effector T cells. Importantly, we were able to extend graft survival using a subtherapeutic dose of tacrolimus combined with β2‐AR agonist. Together, these data reveal a feasible pathway, which, following further pre‐clinical optimization, can be tested in patients receiving VCA or other types of allotransplants.
2. MATERIALS AND METHODS
2.1. Mice
Female C57BL/6 (H‐2b), C57BL/6 (H‐2b, CD45.1) and BALB/c (H‐2d) mice aged 7–8 weeks were purchased from Charles River (Kingston, NY) and The Jackson Laboratory (Bar Harbor, ME) as recipients and donors, respectively. β2‐AR KO mice on BALB/c and C57BL/6 background are bred in‐house from an established colony. Mice were fed a standard laboratory diet and housed under standard light and accommodation conditions. All animal experiments were done with the approval of Roswell Park Comprehensive Cancer Center Animal Care and Use Committee IACUC.
2.2. VCA surgery
All procedures were carried out under sterile conditions by one investigator (M.K.) as described in our previous published work. 37 Briefly, a donor's abdominal aorta and femoral vein were used for revascularization with a recipient's common carotid artery and external jugular vein, respectively, using a non‐suture cuff technique. We used BALB/c background strain as donors and C57BL/6 background strain as recipients because we have revealed that a BALB/c strain had a higher anatomical mutation rate on the Circle of Willis than C57BL/6 strain. 37
2.3. Drug treatments
Immunosuppression was induced in mice using tacrolimus (Sigma‐Aldrich, St. Louis, MO) in doses of 2 or 4 mg/kg (in DMSO; Sigma‐Aldrich, St. Louis, MO) injected subcutaneously with a micro syringe (Hamilton, Reno, NV) daily. The 15 µg/µl of tacrolimus concentration was prepared, and up to 6 µl of diluent was injected without notable toxicity. β2‐AR activation was achieved using daily intraperitoneal injections of 2‐mg terbutaline or .05‐mg bambuterol (200 µl in DPBS; Corning Inc., Corning, NY). The same DPBS was used for vehicles.
2.4. HR and BP measurement
A noninvasive blood pressure (BP) monitoring system (CODA, Kent Scientific Corporation, Torrington, CT) was used to measure heart rate (HR) and BP in mice. 38 Mice were acclimated with the system for 10 days prior to initiating experimental measurements. The results were recorded 6 h after each β2‐agonist injection during the period of experiment.
2.5. Blood collection
Blood was collected from the right superficial temporal vein (STV) using a sterile 5‐mm animal lancet (Medipoint, Inc., Mineola, NY) after anaesthesia induction. The STV is a large vessel positioned posterior to the eye, which can be traced one eye length back and one eye width up from the sebaceous gland. 39 Concentrations of tacrolimus were measured in plasma prepared from the blood samples, which were collected 24 h after previous tacrolimus injection by VITROS 5.1 FS (Ortho Clinical Diagnostics, Inc., Rochester, NY).
2.6. H&E and IHC staining
Following standard euthanasia, grafted tissue was harvested and fixed in 10% formaldehyde (Thermo Fisher Scientific, Waltham, MA), and then tissue was embedded in paraffin. Formalin fixed paraffin sections were cut at 4 µm, placed on charged slides and dried at 60°C for 1 h. Slides were cooled to room temperature and added to the Leica Bond RX, where they were deparaffinized with Bond Dewax Solution (Leica, Allendale, NJ) and rinsed in water. Bond Epitope Retrieval Solution 2 (Leica, Allendale, NJ) was used for target retrieval for 30 min. Slides were blocked using peroxide block from a Bond Polymer Refine Detection kit (Leica, Allendale, NJ) for 5 min. Slides were incubated with CD4 Antibody (Abcam, Cambridge, United Kingdom) at 1/1000 or CD8 (Abcam, Cambridge, United Kingdom) at 1/1000 or FOXP3 (Boster Biological Technology, Pleasanton, CA) at 1/50 for 20 min followed by Rabbit Envision (Agilent Technologies, Santa Clara, CA) for 30 min. Diaminobenzidine from the Bond Polymer Refine Detection kit (Leica, Allendale, NJ) was applied for 10 min for visualization. Slides were counterstained with haematoxylin from the Bond Polymer Refine Detection kit (Leica, Allendale, NJ) for 8 min then placed into water. After removing slides from the Bond they were dehydrated, cleared and cover‐slipped.
2.7. Immunofluorescence histology
OCT (Sakura Finetek, Tokyo, Japan)‐embedded tissue cryosections (9‐µm thick) were fixed at −20°C in methanol/acetone (3:1), blocked using 1% bovine serum and stained with primary antibodies anti‐mouse ICAM‐1, ICAM‐2, vascular cell adhesion molecule‐1 (VCAM)‐1 antibodies (BD Biosciences, San Jose, CA) and anti‐mouse CD31 antibody (Abcam, Cambridge, United Kingdom). Images of at least five consecutive fields (unit area of each field, .34 mm2) were captured by observers blinded to sample identity. Identical exposure times and image settings were used within each experiment. Images were analysed with ImageJ software (NIH, Bethesda, MD) for the determination of the relative fluorescence staining intensity; regions of interest were defined based on CD31 fluorescence, and each pixel in identified regions was assigned a fluorescence intensity value (based on a scale from 0 to 255).
2.8. Flow cytometry
Spleens were mechanically disrupted and directly passed through a 70‐µm nylon cell strainer (Alkali Scientific, Pompano Beach, FL) followed by lysing red blood cells with hypotonic lysis buffer (Gibco, Gaithersburg, MD). Single‐cell suspensions were created from whole tissue transplanted grafts using the Medimachine tissue disruption system (Becton, Dickinson, Franklin Lakes, NJ), followed by leukocyte isolation using Lymphoprep (Stemcell Technologies, Vancouver, Canada). Prepared cells were stained with different antibodies for extracellular and intracellular markers. Antibodies of CD45 (BUV395, clone; 30‐F11), CD45.1 (BUV395, clone; 20), CD45.2 (BV605, clone; 104), CD3 (Alexa Fluor 700, 17A2), CD4 (PerCp, clone; RM4.5), CD8 (Alexa Fluor 488, clone; 53‐6.7), CD25 (APC, clone; PC61), CD44 (V450, clone; IM7), CD62L (PE, clone; MEL‐14), Foxp3 (PE, clone; MF23), IFN‐γ (PE‐CF594, clone; XMG1.2), IL4 (APC, clone; 11B11) and IL17 (BV421, clone; TC11‐18H10) were used (BD Bioscience, San Jose, CA). Golgi stop, fixation and Permeabilization Kit (BD Bioscience, San Jose, CA) were used for staining intracellular cytokines. All data were collected on an LSRFortessa flow cytometer (BD Biosciences, San Jose, CA) and analysed with WinList 9.0 software (Verity Software House, Topsham, ME). The markers CD44 and CD62‐L were used to classify CD4+ and CD8+ T cells as naive (CD44− CD62‐L+), central memory (CM) (CD44+ CD62‐L+) or effector (CD44+ CD62‐L−). 40 , 41 The gating strategies for flow cytometry were represented in Figure S1.
2.9. Luminex assay
Plasma was prepared from collected blood after a 20‐min centrifuge at 800 g without using a brake. Mouse 11‐plex cytokine and 9‐plex chemokine were performed by Flow and Imaging Cytometry Shared Resource, Luminex Division at Roswell Park Comprehensive Cancer Center per the manufacturer's instructions (Invitrogen, Carlsbad, CA).
2.10. Bone marrow chimeras
Chimeras were generated between BALB/c WT and β2‐AR KO mice as donors. Recipient mice were lethally irradiated with 8.0 Gy of total body irradiation (Cesium137 source). One day after irradiation, bone marrow (BM) was reconstituted with the intravenous injection via a tail vein of 10 × 106 donor cells. Reconstituted mice were used 8 weeks after BMT.
2.11. Assessment of rejection grade
Gross rejection grades and pathology rejection grades were evaluated by M.K. and P.N.B., respectively, based on the Banff 2007 working classification of skin‐containing composite tissue allograft pathology. 42
2.12. Statistical analysis
Comparisons between groups were performed using Student's t test, and statistical significance was accepted with p < .05. Also, Two‐way ANOVA was performed to compare the change of measurements over time between groups by testing the group by time interaction effects. Note that a only small number of following pairwise comparisons were conducted, which were pre‐planned and mutually complementary, so correction for multiple testing was not necessary. In addition, Pearson's correlation was used with 95% confidence interval to deliver p values in the correlation data. Log‐rank (Mantel–Cox) test was used for survival comparisons between groups using GraphPad Prism, Version 8 software (GraphPad Software, Inc., La Jolla, CA).
3. RESULTS
3.1. Safety and cross‐reactivity with tacrolimus of selective β2‐AR agonist
Our previous work has established a reliable and consistent platform using a pre‐clinical murine model of hind limb VCA 37 to investigate novel therapies to prolong transplant survival. The transplanted graft consisted of skin, fat, muscle, bone and blood vessels (Figure S2A), a complex combination of tissues similar to those which are often used in VCA.
Although we know from the literature and its clinical safety profile that terbutaline is considered to be a safe drug, we wanted to be sure that there were no cardiac physiology problems generated by this β2‐AR agonist alone, or in combination with the drug tacrolimus, a drug that causes significant immunosuppression and commonly used in the transplant setting. 14 , 43 We assessed BP and HR with a non‐invasive tail cuff system. 44 Pre‐transplanted recipient mice were acclimated to the device for 10 days prior to collecting readings. No statistical difference was detected in systolic/diastolic BP or HR between mice treated daily with β2‐AR agonist or vehicle over a course of 11 days (Figure S2B). We also measured plasma levels of tacrolimus in the mice treated with two different doses; full dose (fTac, i.e. a dose known to generally maintain allografts long term 37 ) and half dose (hTac, i.e. a dose that only delays graft rejection) were measured. As expected, significantly higher drug concentrations were measured in the fTac injected group compared to the hTac group, but there was no significant difference in tacrolimus concentrations between mice treated with tacrolimus alone and mice treated with tacrolimus and β2‐AR agonist combination (Figure S2C). In addition, no changes of body weight and physical appearance were detected suggesting that it was safe to use terbutaline alone and in combination with tacrolimus without drug interaction.
3.2. Decreased rejection responses and T‐cell infiltration into grafts with β2‐AR agonist alone and in combination with tacrolimus
Next, we assessed the impact of β2‐AR agonist on VCA survival by evaluating the impact of terbutaline in recipients employing histological haematoxylin and eosin (H&E) and immunohistochemistry (IHC) stains performed on transplanted grafts 5, 7 and 10 days after VCA to measure rejection grades and CD4+ T, CD8+ T and Foxp3+ cell infiltration into grafts.
Gross and histologic VCA rejections were graded using the Banff 2007 working classification of skin‐containing composite tissue allograft pathology (Figure 1A,B). 42
FIGURE 1.
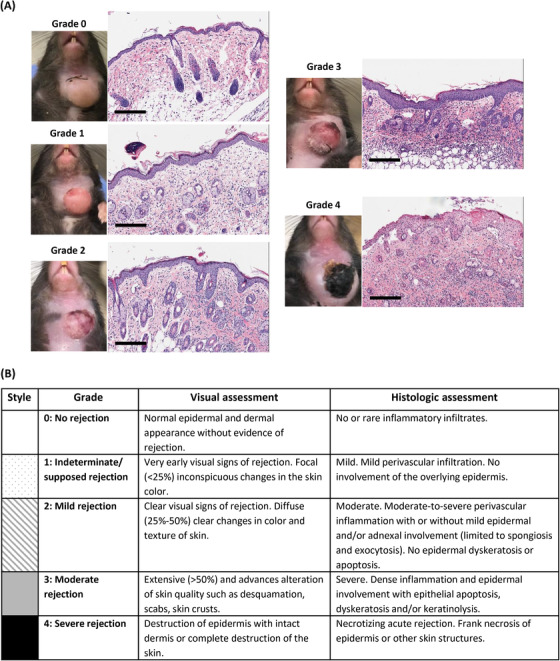
Visual and histologic grading systems for assessment of rejection after vascularized composite tissue allotransplantation (VCA). (A) Examples of each clinical and histologic rejection grade in a murine heterotopic hind limb transplant performed in a total major histocompatibility complex (MHC)‐mismatch. Scale bar: 50 µm. (B) The Banff 2007 working classification of skin‐containing composite tissue allograft pathology
Reduced lymphocyte infiltration into the graft at an early phase of rejection would be expected to correlate with the observed enhanced graft survival. Although moderate perivascular inflammation was detected at day 5 in the β2‐agonist‐treated group (pathology grade 2), epithelial apoptosis and dyskeratosis were distinct in the vehicle‐treated group (pathology grade 3) (Figures 2A,B and S3A,B). Infiltrating lymphocytes (CD4+ T, CD8+ T and Foxp3+ cells) were concentrated in the donor, but not recipient tissue, in both vehicle (Figures 2A, S4A–S6A) and β2‐agonist (Figures 2B, S4B–S6B)‐treated mice; however, fewer lymphocytes infiltrated the grafts in the β2‐agonist group (Figure 2C). Although significantly less T‐cell infiltration was detected in β2‐agonist treated grafts, compositions of CD4+/CD8+ CM and effector memory (EM) T‐cell populations did not show a statistical difference by flow cytometry 5 days after VCA in the grafts (Figure 2D). By day 7, the histological delineation between epidermis and dermis was lost and tissue rejection was nearly complete in the vehicle (Figure S3C) compared to the β2‐agonist group (Figure S3D) and infiltrated CD4+ T, CD8+ T and Foxp3+ cells found in vehicle controls were declining resulting in no statistical difference with the β2‐agonist‐treated group (Figure S3E). The majority of T cells in the grafts 7 days after VCA were EM cells in both groups, and these values were higher than healthy donor (pre‐transplanted grafts) control specimens (Figure S3F).
FIGURE 2.
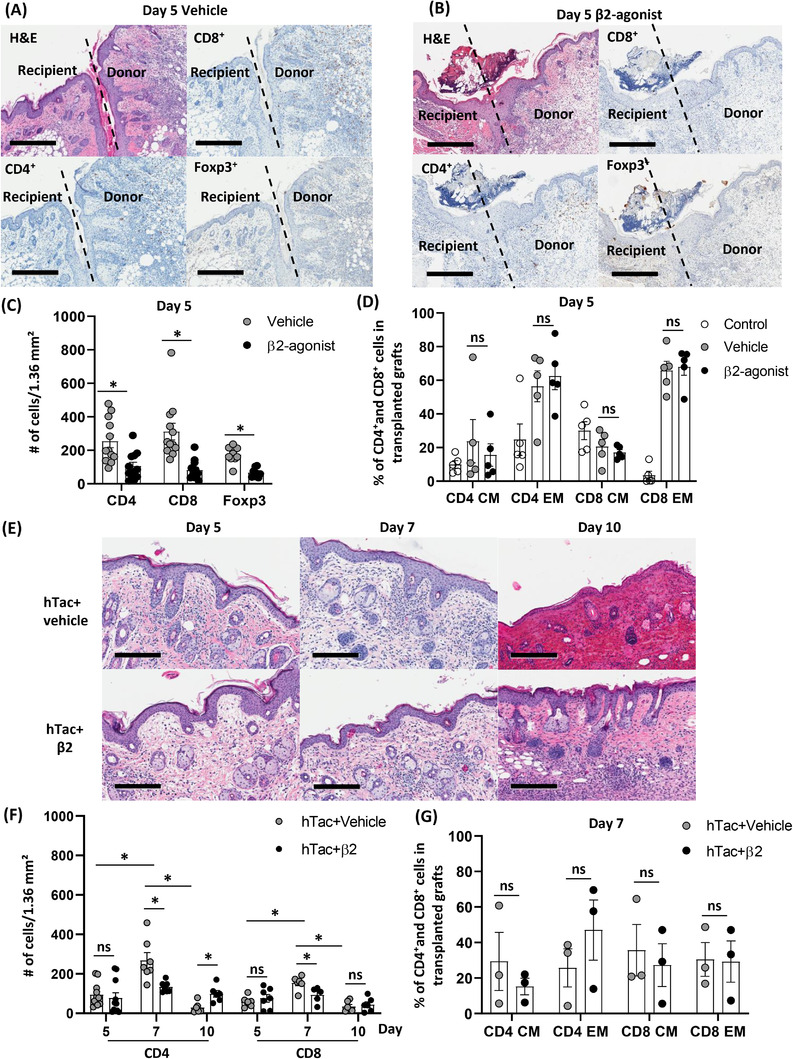
β2‐Adrenergic receptors (ARs) agonist decreases T‐cell infiltration in transplanted grafts along with lower numbers of Foxp3 positive cells compared to the vehicle‐injected group. (A and B) Representative figures for haematoxylin and eosin (H&E) and immunohistochemistry (IHC) with CD8, CD4, and Foxp3 antibodies with either vehicle or β2‐agonist treatment for 5 days. The borderline (black dotted line) differentiates recipient and donor, scale bar: 400 µm. (C) Numbers of CD4, CD8 and Foxp3 positive cells in grafts 5 days after vascularized composite tissue allotransplantation (VCA). Over eight fields from three mice/group. Error bar, standard error of the mean. *p < .05 by Student's t test. (D) Compositions of CD4+/CD8+ central memory (CM) and effector memory (EM) T‐cell populations in transplanted grafts 5 days after VCA. Control, non‐vascularized donor grafts; n = 5. ns, not significant; error bar, standard error of the mean. *p < .05 by Student's t test. (E) Representative figures for H&E stain at different time points after VCA. Scale bar: 200 µm. (F) Numbers of CD4+ and CD8+ T cells in grafts 5, 7 and 10 days after VCA in the hTac + vehicle and the hTac + β2‐agonist‐treated groups. Over five fields from n = 3/group. Error bar, standard error of the mean. *p < .05 by Student's t test. (G) Compositions of CD4+/8+ CM and EM T‐cell populations in transplanted grafts 7 days after treatment either with hTac or hTac + β2‐agonist. n = 3. ns, not significant; error bar, standard error of the mean
To test whether we could use a β‐AR agonist to mimic natural NE and β‐AR interactions and reduce the dose of tacrolimus needed for immunosuppression, we tested tacrolimus at half dose (hTac) in combination with either vehicle or β2‐agonist. Grade 4 graft rejection was observed 10 days after VCA in the hTac + vehicle group, whereas the hTac + β2 group showed mainly only grade 2 with partial grade 3 rejection with intact skin histology (Figure 2E). Abundant CD4+ T and CD8+ T‐cell infiltration was present 7 days after VCA in the hTac + vehicle group, and then the values dropped significantly 3 days later. β2‐Agonist treatments significantly decreased the number of infiltrating CD4+ T and CD8+ T cells at day 7 compared to the hTac + vehicle group, but more CD4+ T‐cell infiltration was found in the β2‐agonist‐treated group than the vehicle injected group at day 10 representing remnant immune responses in the graft (Figure 2F). There was no statistical difference in the proportion of CM and EM T cell populations in grafts 7 days after VCA between two groups using hTac (Figure 2G). Significant interaction effects by two‐way ANOVA also suggest that increase in the number of infiltrating CD4+ T and CD8+ T cells from day 5 to 7 is attenuated by the β2‐agonist treatments (Table S1). This finding demonstrated that β2‐agonist significantly decreased T‐cell infiltration in transplanted grafts compared to vehicle injection after VCA, which is the likely basis for prolonged survival of the grafts.
3.3. Changes in memory T and Th1 cells with a β2‐AR agonist treatment in recipients
It was important to clarify the origin of the infiltrated lymphocytes in the transplanted grafts for applying a target therapy. To determine whether infiltrating cells originated from the donor or the recipient, we took advantage of differential CD45 isoform usage of C57BL/6 recipient (CD45.1) and BALB/c donor (CD45.2) mice. By day 7 post‐VCA, over 90% of leukocytes within grafts (Figure S7A) and peripheral blood (Figure S7B) were from recipients.
The frequency of different lymphocyte populations was analysed with either vehicle or β2‐agonist injections to investigate effects on recipients. In contrast to observations within grafts by flow cytometry (Figure 2D,G), β2‐AR agonist treatment significantly decreased the representation of CD4+ and CD8+ memory (CM and EM) T‐cell populations in the recipient's systemic blood compartment (spleen) at day 5 (i.e. before the emergence of signs of rejection; Figure 3A); however, by day 7 (i.e. once gross signs of rejection are apparent) these differences were lost (Figure 3B). This finding was more pronounced in the EM T‐cell population in mice treated with hTac. Additional decreases in the CD4+ T and CD8+ T EM populations were found with β2‐agonist treatment at day 7 (Figure 3C), and these differences are lost by day 10 (Figure 3D). β2‐Agonist decreased the compositions of Th1 population significantly compared to the vehicle group at day 5 and 7 without tacrolimus (Figure 3E) and at day 7 and 10 with hTac (Figure 3F). Interestingly, higher Treg (CD4+CD25+Foxp3+) levels in the graft (Figure 2C) and the body (spleen; Figure 3E) did not predict a better prognosis for transplanted grafts. There were more Foxp3+ cell infiltration in grafts and a greater Treg population systemically along with more infiltrated T cells in grafts. The Treg population was relatively proportional to the severity of T‐cell infiltration in grafts after VCA (Figure S8A,B). Accordingly, a smaller Treg population was found in grafts and the recipients treated with fTac (Figure S8C,D). Thus, Treg populations, which are thought to have immunosuppressive effects, may have increased as a consequence of a fulminant immune response to the highly antigenic VCA.
FIGURE 3.
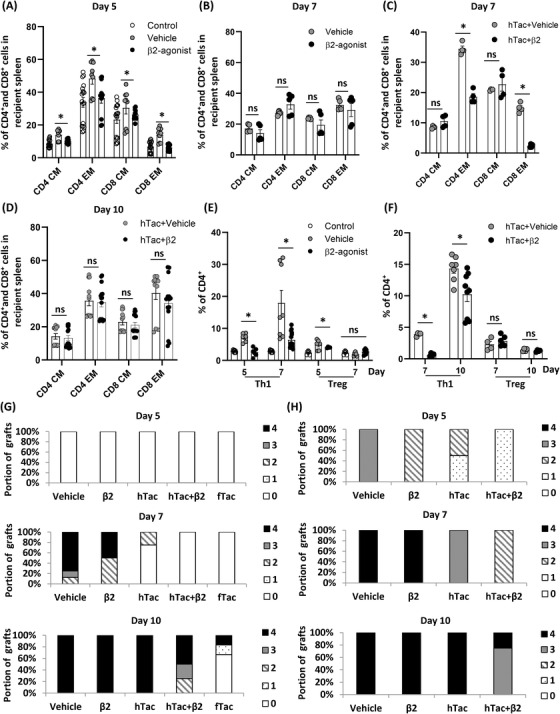
β2‐Adrenergic receptor (AR) agonist decreases CD4+/CD8+ effector memory (EM) T‐ and Th1‐cell populations and significantly decreases cytokine levels such as IFN‐γ, IL‐6 and TNF‐α compared to the vehicle group in recipients’ blood. (A and B) Compositions of CD4+/CD8+ central memory (CM)/EM T‐cell populations in the blood 5 and 7 days either with vehicle or β2‐agonist after vascularized composite tissue allotransplantation (VCA) without tacrolimus. Day 5, n ≥ 9; day 7, n = 5; control, non‐transplanted animals. ns, not significant; error bar, standard error of the mean. *p < .05 by Student's t test. (C and D) Compositions of CD4+/CD8+ CM/EM T‐cell populations in the blood 7 and 10 days with either vehicle or β2‐agonist after VCA with a half dose of tacrolimus (hTac). Day 7, n = 4; day 10, n ≥ 12. ns, not significant; error bar, standard error of the mean. *p < .05 by Student's t test. (E and F) Th1‐ and Treg‐cell populations in CD4+ T cells without and with tacrolimus after VCA with either vehicle or β2‐agonist treatments. Day 7, n ≥ 6; day 10, n ≥ 4. ns, not significant; error bar, standard error of the mean. *p < .05 by Student's t test. (G) Gross (clinical) rejection grades depending on different treatments after VCA. Vehicle, n = 8; β2, n = 4; hTac, n = 4; hTac + β2, n = 4; fTac, n = 6. (H) Histologic rejection grades depending on different treatments after VCA, n = 4/group
No gross rejection was observed in any graft prior to 5 days post‐VCA, but over 85% of the vehicle injected recipients showed grade 3 or 4 rejection 7 days after VCA. In contrast, β2‐AR‐agonist‐treated recipient mice had less severe rejection (grade 2) at day 7, which was further improved by the addition of subtherapeutic dose of tacrolimus (hTac), with some grafts surviving with grade 2 rejection at day 10 (Figure 3G). Although no evidence of gross rejection was observed at day 5, various histologic rejection grades were detected with H&E. β2‐Agonist treatments delayed rejection responses with/without subtherapeutic dose of tacrolimus compared to the vehicle‐injected group (Figure 3H). The data demonstrate that stimulation of β2‐AR decreases the presence of effector T‐ and Th1‐cell populations in recipients along with delayed visual and histologic evidence of rejection, and more Foxp3+ cells appear when more T cells exist in the graft representing severe rejection responses after VCA.
3.4. Analyses of inflammatory cytokine levels after β2‐AR modulation in recipients
The helper T cell (Th) is one of predominant populations releasing cytokines. 45 β2‐Agonist decreased the frequency of Th1 significantly, but it was important whether manipulating of β2‐AR with agonists suppressed the production of cytokines. Luminex analyses were used to determine if differences in the expression of inflammatory cytokines are associated with graft rejection after VCA followed by vehicle or β2‐agonist injections. Systemic cytokines in recipient's plasma were measured. Significantly decreased IFN‐γ, IL‐6 and TNF‐α levels with increased IL‐13 levels were found in the β2‐agonist group compared to the vehicle group 5 days after VCA. The elevated cytokines (IFN‐γ, IL‐6, IL‐18 and TNF‐α) decreased 2 days later was indicative of their likely role in graft rejection responses before phenotypic rejection was noted (Figure 4), and the two‐way ANOVA showed that changes of cytokine levels from day 5 to 7 were statically different between two groups in IFN‐γ, IL‐6, IL‐13 and TNF‐α (Table S2). This finding suggests that β2‐agonist decreases the production of proinflammatory and inflammatory cytokines in recipients after VCA.
FIGURE 4.
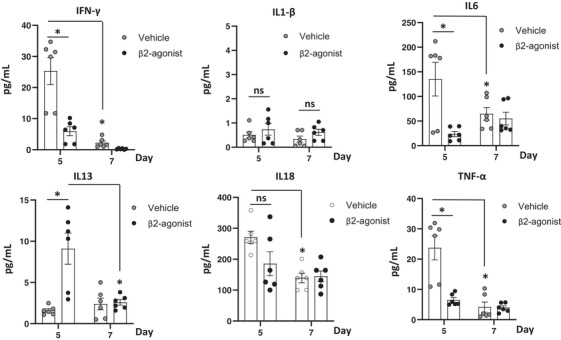
β2‐Adrenergic receptor (AR) modulation decreases inflammatory cytokine productions in recipients. In the setting of wild‐type (WT) donors and WT recipients vascularized composite tissue allotransplantation (VCA) without tacrolimus, various cytokine levels were analysed 5 and 7 days after transplant by Luminex assay. n = 6; pooled data from duplicate samples. Error bar; standard error of the mean. *p < .05 by Student's t test
3.5. Augmentations of subtherapeutic immunosuppression using tacrolimus in combination with β2‐AR agonist
VCA recipients often must accept a reduction or a cessation of immunosuppressive drugs after various lengths of time because of toxic side effects. We investigated whether addition of β2‐agonist could allow a reduced dose of a standard immunosuppressive drug such as tacrolimus. Two different β2‐AR agonists as a short‐ and long‐acting compounds with terbutaline and bambuterol, respectively, were evaluated for their effects on the survival of grafts after cessation of immunosuppression. Recipients were given with fTac every day for 14 days, and then tacrolimus injection was stopped, and vehicle or β2‐agonist injections were initiated and continued until grafts showed rejection (Figure 5A). Distinct effects of a short‐acting β2‐agonist treatment, terbutaline, on suppression of memory T‐cell populations were not detected, but a long‐acting β2‐agonist, bambuterol, suppressed the compositions of CD4 EM and CD8 CM populations significantly compared to the vehicle group in the blood 21 days after transplant (Figure 5B). Even though there was no survival benefit of grafts given a short‐acting β2‐agonist following cessation of tacrolimus (Figure 5C), bambuterol improved graft survival significantly compared to other two groups (Figure 5C). In addition, with a scenario using hTac with either vehicle or β2‐agonists (Figure 5D), the dose of tacrolimus was reduced by half 14 days after a full dose of tacrolimus daily treatment (fTac to hTac). Even though no statistical difference on CD4+ and CD8+ memory T‐cell populations was detected between three groups in the blood 28 days after transplant (Figure 5E), there was a significant graft survival benefit in the β2‐agonist groups using a short‐ and long‐acting compounds compared to the vehicle group (Figure 5F). These studies reveal that although a β2‐agonist cannot replace a conventional immunosuppressive drug, tacrolimus, as a single agent, their addition is able to extend graft survivals and delay rejection responses when recipients are not given the full dose of tacrolimus.
FIGURE 5.

β2‐Adrenergic receptor (AR) agonist injected group achieves significantly longer graft survival than the vehicle injected group. (A) Wild type (WT) recipients having WT donor grafts were treated with a full dose (optimal) of tacrolimus (fTac) for 14 days and then treated with either vehicle or β2‐agonists (terbutaline and bambuterol) after cessation of fTac. (B) CD4+/CD8+ central memory (CM) and effector memory (EM) T‐cell compositions were analysed in the blood 21 days after vascularized composite tissue allotransplantation (VCA), 7 days after treatment with either vehicle or β2‐agonist. n = 5. ns, not significant; error bar, standard error of the mean. *p < .05 by Student's t test. (C) Graft survival curves. n = 5. ns, not significant. *p < .05 by log‐rank test using GraphPad Prism. (D) WT recipients were treated with a half dose (subtherapeutic) of tacrolimus along with vehicle or β2‐agonists (terbutaline and bambuterol) after 14‐day fTac injections. (E) CD4+/CD8+ CM and EM T‐cell compositions were analysed in the blood 28 days after VCA, 14 days after treatment with either vehicle or β2‐agonist. n = 5. ns, not significant by Student's t test; error bar, standard error of the mean. (F) Graft survival curves. n = 5. *p < .05 by log‐rank test with GraphPad Prism
3.6. Effects of β2‐AR modulation in donor tissue
β2‐AR normally exists in both donors and recipients after transplant, so it was necessary to investigate whether β2‐agonist activated AR signals on donors’ or recipients’ cells to delay rejection responses. Well‐studied AR KO mouse strains as donors and recipients were used to investigate the specific role of β2‐AR in the treatments of either donor or recipient. 34 Grafts were harvested from BALB/c AR KO mice as donors, and transplanted into C57BL/6 WT recipients (Figure 6A–D). Although the graft showed less severe gross rejection as grade 1 with β2‐agonist compared to the vehicle injection (Figure 6A), no statistical difference was found in the compositions of CD4+ and CD8+ memory T‐cell populations at day 5 in the spleen (Figure 6B). However, the composition of CD4+ and CD8+ EM T‐cell populations were significantly lower in the β2‐agonist group than the vehicle group at day 7 (Figure 6C), and significantly less Th1 and Treg were detected with β2‐agonist compared to vehicle at day 5 (Figure 6D). In parallel, WT grafts were transplanted to C57BL/6 AR KO recipients with either vehicle or β2‐agonist injections (Figure 6E‐H). Significant decreases in CD4+ and CD8+ memory T‐cell populations were detected at day 5 (Figure 6F) and 7 (Figure 6G) with gross grade 0 rejection in the β2‐agonist‐treated group compared to the vehicle group (grade 3). In addition, β2‐agonist decreased Treg population significantly compared to vehicle injection at day 5 (Figure 6H). Furthermore, significant decreases in CD4+ T cell and Treg infiltration were detected with β2‐agonist treatment in AR KO recipients bearing the WT grafts (WT → KO) compared to WT recipients bearing AR KO grafts (KO → WT) (Figure 6I). Although the infiltrated lymphocytes in grafts originated from recipients (Figure S3), the manipulation of donor's β2‐AR signals is critical for suppressing T‐cell infiltration and rejection responses.
FIGURE 6.
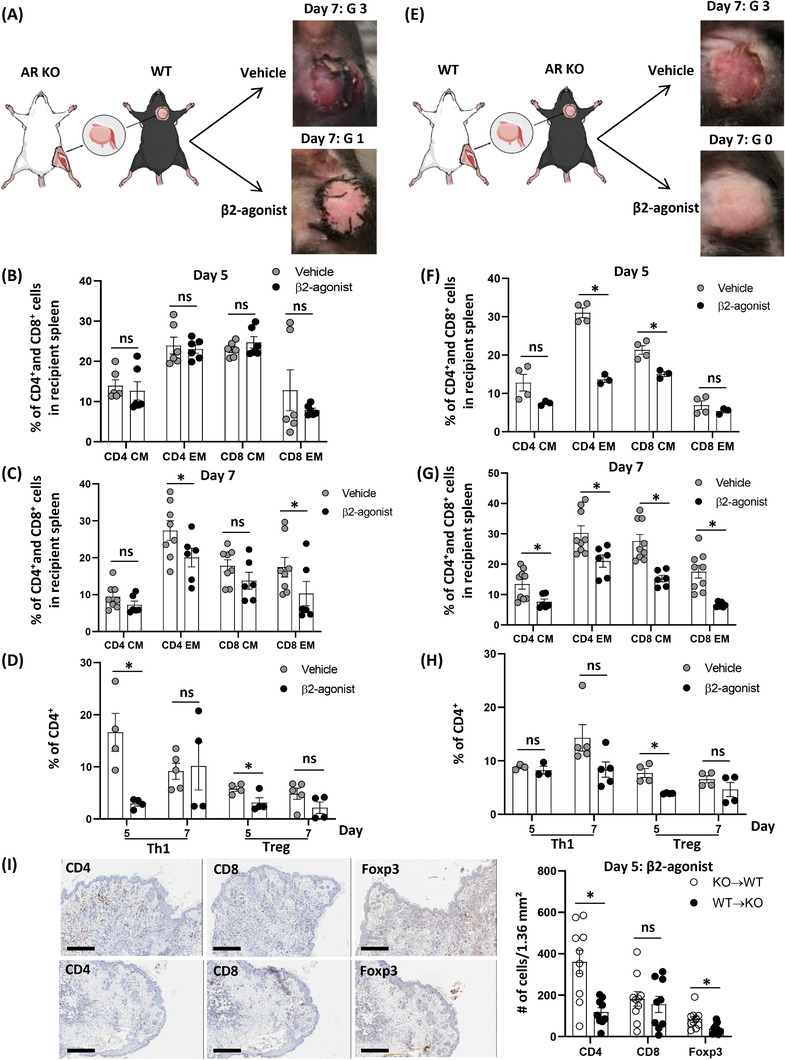
Modulation of β2‐adrenergic receptor (AR) signalling in donors is more effective to delay rejection responses than the modulation in recipients. (A) BALB/c β2‐AR knock‐out (KO) donors were transplanted to C57BL/6 recipients with either vehicle or β2‐agonist injections. (B and C) Compositions of CD4+/CD8+ central memory (CM)/effector memory (EM) T‐cell populations in the spleen 5 and 7 days with either vehicle or β2‐agonist after vascularized composite tissue allotransplantation (VCA) without tacrolimus in wild‐type (WT) recipients receiving BALB/c β2‐AR KO grafts. Day 5, n = 6; day 7, n ≥ 6. ns, not significant; error bar, standard error of the mean. *p < .05 by Student's t test. (D) Th1‐ and Treg‐cell populations in CD4+ T cells after vehicle or β2‐agonist treatments in WT recipients receiving BALB/c β2‐AR KO grafts. n ≥ 4. ns, not significant; error bar, standard error of the mean. *p < .05 by Student's t test. (E) BALB/c WT donors were transplanted to C57BL/6 β2‐AR KO recipients with either vehicle or β2‐agonist injections. (F and G) Compositions of CD4+/CD8+ CM/EM T‐cell populations in the spleen 5 and 7 days with either vehicle or β2‐agonist after VCA without tacrolimus in β2‐AR KO recipients receiving BALB/c WT grafts. Day 5, n ≥ 3; day 7, n ≥ 6. ns, not significant; error bar, standard error of the mean. *p < .05 by Student's t test. (H) Th1‐ and Treg‐cell populations in CD4+ T cells with either vehicle or β2‐agonist treatments in β2‐AR KO recipients receiving BALB/c WT grafts. n ≥ 3. ns, not significant; error bar; standard error of the mean. *p < .05 by Student's t test. (I) Representative figures for immunohistochemistry (IHC) with CD4, CD8 and Foxp3 antibodies with β2‐agonist treatment for 5 days in WT recipients bearing AR KO donor's grafts (KO → WT) and AR KO recipients bearing WT donor's grafts (WT → KO). Over nine fields from n = 3/group. ns, not significant; error bar, standard error of the mean; scale bar: 400 µm. *p < .05 by Student's t test
3.7. Important actions of β2‐AR agonist on donor stromal cells after VCA
Since our VCA model contains a donor's femur with BM, it was important to discriminate the effects of β2‐agonist on hematopoietic and stromal cells in transplanted grafts. β2‐AR modulation in donors suppresses rejection responses after VCA, so to better determine the responsible populations of cells, BM and stromal cells of the donor were examined. WT donors were exposed to 8‐Gy irradiation the day before VCA to eradicate BM cells, and then grafts, including a femur, were transplanted to β2‐AR KO recipients followed by vehicle or β2‐agonist injections. Both groups showed mild xerosis of grafts with intact skin anatomy (Figure 7A), and distinct effects of β2‐agonist treatment were lost in the CD4+ and CD8+ memory T cell (Figure 7B), Th1 and Treg (Figure 7C) populations 7 days after VCA. However, significantly lower numbers of CD4+ and CD8+ T‐cell infiltrations were found in the β2‐agonist‐treated graft than the vehicle group (Figure 7D). Further, a β2‐AR KO donor was irradiated, and then WT BMT was performed to generate a chimeric model, including β2‐AR KO stromal cells and WT BM, and vice versa (Figure 8A). Grafts composed of β2‐AR KO stromal with WT BM (KO + WT) showed more severe rejection than WT stromal with AR KO BM (WT + KO) grafts in AR KO recipients (Figure 8A,B; KO + WT gross grade 2 and histologic grade 3, WT + KO gross grade 1 and histologic grade 2). Greater amounts of CD4+ T and CD8+ T‐cell infiltration were found in the KO + WT grafts than the WT + KO grafts (Figure 8B). Additionally, significant decreases of CD4+ and CD8+ memory T‐cell populations were detected in the systemic immune responses of mice bearing WT + KO grafts compared to mice bearing KO + WT grafts (Figure 8C) with no significant difference on Th1 and Treg populations between groups (Figure 8D). It means that β2‐AR signals in donor's stromal cells have critical roles on recipient's T‐cell trafficking.
FIGURE 7.
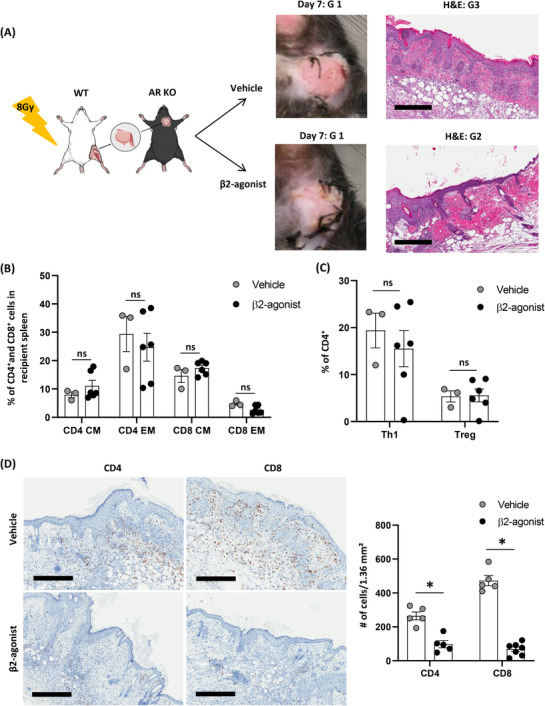
β2‐Adrenergic receptor (AR) agonist has effects with donor's stromal cells to inhibit T‐cell trafficking into transplanted grafts. (A) BALB/c wild‐type (WT) mice as a donor were exposed to 8‐Gy irradiation the day before surgery, the composite graft was transplanted to C57BL/6 β2‐AR knock‐out (KO) recipients with either vehicle or β2‐agonist treatments without tacrolimus. (B) Compositions of CD4+/CD8+ CM and (C) effector memory (EM) T‐cell populations and Th1 and Treg populations. n ≥ 3. ns, not significant; error bar, standard error of the mean. *p < .05 by Student's t test. (D) Immunohistochemistry (IHC) with CD4/CD8 antibodies, and cells were counted by ImageJ programme. Over five fields from n = 3/group. ns, not significant; error bar, standard error of the mean; scale bar: 400 µm. *p < .05 by Student's t test
FIGURE 8.
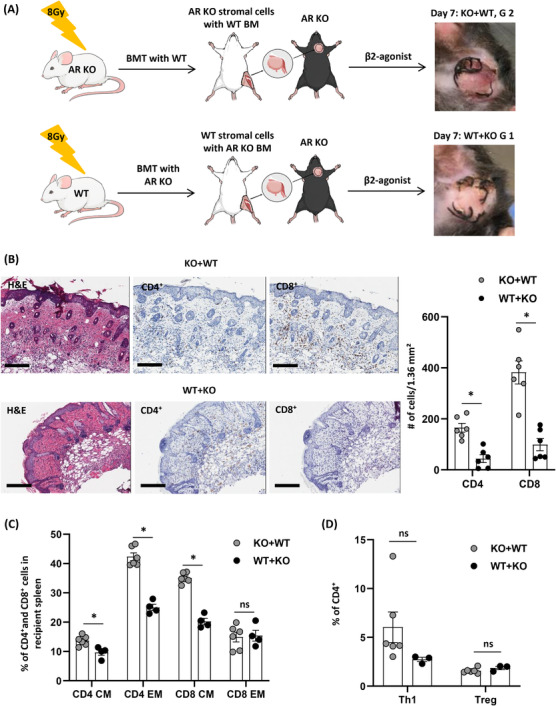
β2‐Adrenergic receptor (AR) agonist has effects with donor's stromal cells to inhibit T‐cell trafficking into transplanted grafts. (A) BALB/c AR knock‐out (KO) and wild‐type (WT) donors were exposed to 8 Gy irradiation, and then BALB/c WT and AR KO bone marrow (BM) were transplanted respectively. After 8‐week bone marrow transplantation (BMT), vascularized composite tissue allotransplantation (VCA) was performed on C57BL/6 AR KO recipients followed by β2‐agonist treatment for 7 days. (B) Haematoxylin and eosin (H&E) and immunohistochemistry (IHC) with CD4 and CD8 antibodies, six fields from n = 3/group. Error bar, standard error of the mean; scale bar: 200 µm. *p < .05 by Student's t test. (C) Systemic compositions of CD4+/CD8+ central memory (CM)/effector memory (EM) T‐cell populations and (D) Th1‐ and Treg‐cell populations in CD4+ T cells 7 days after VCA with β2‐agonist treatment in C57BL/6 AR KO recipients having a graft from either BALB/c β2‐AR KO with WT BMT (KO + WT) or BALB/c WT with AR KO BMT (WT + KO). n ≥ 3. ns, not significant; error bar, standard error of the mean. *p < .05 by Student's t test
3.8. Inhibition of endothelial adhesion molecules and T‐cell trafficking chemokines with β2‐AR agonist
Multiple mechanisms and steps are required for leukocytes to infiltrate into stromal tissue of the donor's graft, and we wondered whether β2‐agonist could change any of these mechanisms. Leukocyte extravasation is one of the essential and first steps during the initiation of cell‐mediated rejection, and ICAM‐1/2 and VCAM‐1 are the endothelial adhesion molecules that mediate firm adhesion just prior to cell extravasation. 46 In addition, chemokines play a central role in directing the migration of leukocytes. 47 To investigate changes of endothelial adhesion molecules in donor's vessels after β2‐agonist treatment, WT donor's grafts in WT recipients were stained 5 days after VCA. β2‐Agonist suppressed ICAM‐1 and VCAM‐1 expression in graft vessels compared to vehicle, whereas the expression of ICAM‐2 was increased in the β2‐agonist‐treated group (Figure 9A) by immunofluorescence. Also, recipient's plasma levels of the relevant T‐cell trafficking chemokines CXCL‐1, CXCL‐10, CCL2, CCL3, CCL4, CCL5, CCL7 and CCL11 increased significantly at day 5, and resolving by day 7. Under β2‐agonist treatment, levels of CXCL‐1, CXCL‐10, CCL3, CCL4, CCL5 and CCL7, which are pro‐inflammatory chemokines that regulates leukocytes trafficking, 48 were decreased significantly compared to the vehicle injection (Figure 9B). This was further supported by the time and treatment interactions in two‐way ANOVA except for CXCL‐2, which suggests that the changes in these chemokine levels are significantly different between treatment groups over time (Table S3). This finding suggests that β2‐agonist manipulated expression levels of endothelial adhesion molecules in donor's stromal cells and suppressed the production of numerous chemokines which were capable of leukocyte trafficking in recipients.
FIGURE 9.
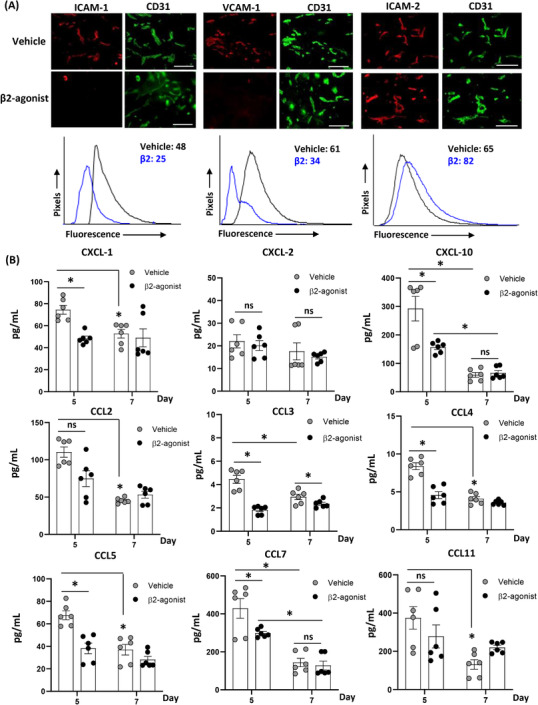
β2‐Adrenergic receptor (AR) agonist suppresses intercellular adhesion molecule‐1 (ICAM‐1) and vascular cell adhesion molecule‐1 (VCAM‐1) expression in donor grafts and significantly decreases CXCL‐1, CCL4 and CCL5 in recipients’ blood. (A) Immunofluorescence (IF) and image quantification were performed 5 days after vascularized composite tissue allotransplantation (VCA) with wild‐type (WT) donors and recipients followed by either vehicle or β2‐agonist injections. ICAM‐1, VCAM‐1, ICAM‐2 and CD31 antibodies were used. Histograms depict quantification of IF intensity of each antibody in all CD31+ vessels; numbers denote micro‐flow imaging (MFI). All data are representative of duplicate experiments, n = 3; scale bar: 100 µm. (B) Using Luminex assay, in the setting of WT donors and WT recipients VCA without tacrolimus, various chemokine levels were analysed 5 and 7 days after transplant. n = 6; pooled data from duplicate samples. ns, not significant; error bar, standard error of the mean. *p < .05 by Student's t test
3.9. Effects of donor preconditioning prior to VCA with β2‐AR agonist
If the same effects of rejection responses are achieved with β2‐agonist treatment in a donor prior to VCA, the application of β2‐agonist can be extended to donors. β2‐Agonist treatment before VCA was examined as a pre‐conditioning regimen as BALB/c WT mice were given β2‐agonist for 2 weeks prior to VCA (pre‐VCA) and compared to the previous experiments injecting β2‐agonist after VCA (post‐VCA) (Figure S9A). Although similar gross and histologic rejection grades were detected in the pre‐VCA group (grades 1 and 3 respectively) compared to the post‐VCA group (grades 0 and 3 respectively) 7 days after surgery, the immunosuppressive effects on memory T‐cell populations in recipients were lost in the setting of pre‐VCA (Figure S9B). But the suppression of the Th1 population and CD8+ T‐cell trafficking in pre‐VCA were comparable to post‐VCA (Figure S9C,D). This experiment reveals that the preconditioning with β2‐AR agonist in donors achieves partial effects of immunosuppression compared to the usage in recipients.
4. DISCUSSION
Vascularized composite allographs are composed of multiple tissues with different immunogenic and functional properties, including skin, muscles, bones and nerves, and they are typically obtained from cadavers. Thus, there is little chance to match HLA types with the recipients. As a result, these donor tissues are highly antigenic requiring very large and toxic doses of drugs such as tacrolimus. 13 , 49 , 50 Here, we tested a strategy that mimics the natural ability of nerves and AR signalling by catecholamines such as NE to suppress anti‐graft T‐cell‐mediated immune responses and prolong survival of a complex tissue allograft. Mimicking the natural (and temporary) suppressive function of nerves on the immune response to promote survival of VCA (or even that of more traditional solid organ transplants) has not previously been tested in either preclinical or clinical settings.
Several groups have tested minimizing the use of immunosuppressants (tacrolimus or steroids) after VCA relying instead on the benefits of steroid‐free immunosuppression in solid organ transplants; unfortunately, this approach has been associated with frequent acute rejection episodes. 51 , 52 Importantly, we found that we could reduce the dose of a standard immunosuppressant drug tacrolimus to half and improve graft survival by augmenting low‐dose tacrolimus with a β2‐AR agonist. It the future, it may be possible that β2‐AR agonists could replace steroids to prevent development of steroid‐related complications, but this remains to be tested. Selective β2‐AR targeting drugs have been safely and extensively used for decades for other indications, particularly for cardiovascular and pulmonary manipulation such as in cases of asthma. 53 In studies on asthma in humans, researchers have even safely used higher doses of terbutaline compared to the recommended dose needed to diminish asthmatic symptoms. 54 At the dose of terbutaline that we used here to prolong graft survival, no changes in HR and BP were observed with daily use, and no drug interaction with tacrolimus was found, thus supporting the safety of this approach.
Mice have a more rapid metabolism than humans, 55 and therefore it is not always possible to draw a direct comparison between the effects of various pharmacological drugs, nor to extrapolate optimal doses from mouse to human. For example, dopamine has been prescribed to treat low BPs in patients. But, low infusion rates act on the visceral vessels to produce vasodilation resulting in increased urinary flow; on the other hand, higher doses cause vasoconstriction and increased BP via the ARs α1, β1 and β2. 56 For these reasons, dose escalation studies of β2‐agonists are needed to optimize future combination regimens with tacrolimus in patients.
Without treatment with β2‐AR agonist, we observed here that CD4+ T and CD8+ T cells were increased significantly in transplanted grafts at the time of rejection. β2‐AR agonist treatment was associated with significantly fewer numbers of infiltrated T cell and decreased EM T and Th1 cell populations in recipients corresponding to the prolonged survival benefit. In addition, we have previously shown that treating stressed mice with β‐AR antagonists alleviated mitochondrial dysfunction, increased glycolysis in CD8+ T cells and increased T‐cell activation resulting in reduced tumour growth rates and significantly fewer exhausted T cells. 57 It is likely that β‐AR agonists may have inhibitory effects on T‐cell activation, and we observed that β‐agonists impaired T‐cell receptor signalling, 57 thus reducing their graft destruction properties. Although several clinical and preclinical studies have shown that Tregs modulate immune responses and that a high level of Treg populations are predictive for a better prognosis after solid organ and haematopoietic cell (HPC) transplantation, 58 in our VCA model increased numbers of Tregs were detected in the presence of strong immune responses after transplant. Thus, recipients with greater levels of Treg populations showed severe gross rejection at early time points and eventual resolution of the population in the recipient's blood and grafts after severe gross rejection was well underway. Although we do not, as yet, understand the basis of this difference, it may be related to the highly antigenic VCA grafts, which contain regions of skin, as opposed to those used in solid organ transplants, particularly at early time points. Organ transplantations have also been associated with increased numbers of immunosuppressive MDSC, 59 , 60 , 61 and as the MDSC population will likely increase with β2‐agonist treatment after VCA (based on β2‐agonist effects in the setting of GVHD after BMT 34 ), these cells may be a more potent contributor to immunosuppression than Treg induction.
In terms of the immune modulatory impact of adding a β2‐AR agonist, terbutaline alone delayed rejection responses in association with significant decreases in CD4+/CD8+ EM T‐ and Th1‐cell populations, cytokines and chemokines such as IFN‐γ, IL‐6, TNF‐α, CXCL‐1/10 and CCL3/4/5/7 in recipients. Addition of the β2‐AR agonist treatment increased the anti‐inflammatory cytokine, IL‐13, which was associated with concomitant down‐regulation of TNF‐α production, a phenomenon observed in other studies. 62 , 63 We found that IL‐18, CCL2 and CCL11 were particularly unresponsive to β2‐agonist treatment indicating that these cytokine/chemokines might be targetable by other agents to further suppress rejection responses after VCA.
β2‐AR treatment of either recipients or donors was sufficient to delay gross rejection responses, but treatment of the donor resulted in a more potent effect. Specifically, β2‐AR treatment of the donor tissue stromal cells was necessary to suppress recipient T‐cell trafficking into grafts. Leukocyte extravasation is a prerequisite for acute rejection in grafts, and their migration into the tissue requires the expression of adhesion molecules on the surface of activated endothelium. 64 , 65 The endothelial adhesion molecules ICAM‐1 and VCAM‐1 are known as the central mediators of leukocyte adhesion to and transmigration across the endothelium, 46 and β2‐AR agonist suppressed ICAM‐1 and VCAM‐1 expression with concomitant decreased T‐cell trafficking into grafts. Of note, ICAM‐2 expression was increased. As previously reported, during longer periods of inflammation, endothelial cells respond to inflammatory mediators by massive up‐regulation of adhesion receptors such as ICAM‐1 and VCAM‐1, whereas ICAM‐2 decreases. 66 , 67 , 68 A possible explanation for our observed increase in ICAM‐2 is that it might represent a compensatory mechanism for decreased ICAM‐1 or an additional effect of β2‐AR modulation. The β2‐AR agonist, terbutaline stimulated angiogenesis on endothelial cells derived from the central nervous system, 69 and ICAM‐2 has been associated with this process. 70 By regulating ICAM‐2 and increasing angiogenesis, β2‐agonist treatment could reduce ischemic injury in harvested organs. Graft salvage effects of β2‐agonist suggest that regulation of endothelial adhesion molecules partially contributes for the prevention of chronic rejection after VCA (Figure 4). Another possible mechanism underlying benefits of a β2‐agonist is that it can increase cAMP (cyclic adenosine monophosphate) levels 71 ; cAMP is known as a potent negative regulator in T cells, which dampens T‐cell‐immune function through the cAMP/protein kinase A signalling pathway. 72
β2‐AR activation in donor BM cells was necessary for the observed immunosuppressive effects, including suppression of IFN‐γ levels in recipients. Although the donor's HPCs were replaced by the recipient's HPC within 5 days after VCA in our model (Figure S2), it is likely that a transient mixed chimerism developed in the recipients, which was sufficient for the induction of allograft tolerance. 73 , 74 , 75 Although the study of transplant tolerance is beyond the scope of this study, β2‐agonist treatment could be a mechanism to facilitate the induction of tolerance and could replace at least some of the more toxic immunosuppressive drugs currently being used.
Interestingly, in a previous study, using allografts from cadaveric kidney donors who had been treated prior to death with dopamine and NE to maintain their BP and HR resulted in reduced acute rejection and improved graft survival after transplantation. 76 , 77 Our current findings suggest a possible mechanism for this observation. β2‐Agonist addition to preservation solutions may be a relatively simple measure to improve organ transplantation, in addition to the treatment of recipients.
One problem with our approach is that terbutaline is a relatively short acting β2‐agonist. It is likely that longer acting agonists (e.g. bambuterol and salmeterol) will be superior to terbutaline, and this assumption is supported by our experiment with bambuterol, a long‐acting β2‐agonist (Figure 5). Another issue is that among our experiments, we noticed some differences in immune subpopulations of animals given the same treatment modality (e.g. Figure 3A,D). Even though the animals were purchased from the same vendor, perhaps small variations in housing factors, or physiological differences in the status of the microbiome, could be a reason for this variation. Published data demonstrate that changes in microbiota may cause different rejection responses after skin transplant. 78 Another limitation is that our study is focused largely upon effector T lymphocytes and their role in graft rejection. Even though current immunosuppressive therapies for solid organ transplantation appear to be dominated by T‐cell mechanisms, β2‐AR exists on B lymphocytes, and innate immune cells such as granulocytes, macrophages, dendritic cells and natural killer cells. 79 , 80 Thus, other immune cell subtypes, which are also associated with graft rejection, may contribute to some of our observed findings, and this should be addressed in future studies. Finally, it will be important to determine whether β2‐agonists can enhance lower doses of other conventional immunosuppressive drugs such as glucocorticoids, alkylating agents, and purine synthesis inhibitors.
5. CONCLUSIONS
There is a significant need to improve graft survival and reduce the doses of toxic immunosuppressant drugs currently being given to patients receiving VCA. We have demonstrated here that by pharmacologically targeting adrenergic stress pathways ordinarily used physiologically by nerves of the autonomic nervous system, we could not only prolong graft survival but more importantly, allow for reduction of the dose of a standard immunosuppressive drug used in various VCA scenarios. We have also identified several relevant mechanisms by which this prolongation of the survival of VCA occurs. Future studies will certainly need to optimize the dose and scheduling of applications of β‐agonists and test additional combinations with immunosuppressive drugs so that the best protocols can be offered to patients receiving VCA in the near future.
CONFLICT OF INTEREST
The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in the article.
Supporting information
FIGURE S1 Gating strategies for flow cytometry
FIGURE S2 A schematic illustration of our vascularized composite tissue allotransplantation (VCA) model and the safety of a selective β2‐adrenergic receptor (AR) agonist drug, terbutaline. (A) BALB/c and C57BL/6 strains were used as donors and recipients respectively. En bloc tissue composed of skin, subcutaneous fat, muscle, vessels and femur was transplanted to a recipient's cervical area. (B) Systolic and diastolic blood pressures (BP) with heart rates (HRs) were measured after everyday injection of with either vehicle (V; PBS) or β2‐agonist (β; terbutaline; 2 mg/day). Mice were acclimated to the BP and HR measuring procedures for 10 days before recording data. Representative data between two different experiments, n = 5. (C) Concentrations of tacrolimus in plasma were analysed 14 days after subcutaneous injections (24 h after the last injection) with either a half dose of tacrolimus (hTac; 2 mg/kg/day) or a full dose of tacrolimus (fTac; 4 mg/kg/day). Representative data between two different experiments, n = 5. ns, not significant; error bar, standard error of the mean. *p < .05 by Student's t test
FIGURE S3 Pathologic findings 5 and 7 days after VCA with either vehicle or β2‐agonist injections. (A and B) Representative haematoxylin and eosin (H&E) images revealed epithelial dyskeratosis (arrowhead) and apoptosis (arrow) in the vehicle‐injected group (A; rejection grade 3) not in the β2‐agonist‐injected group (B; rejection grade 2) 5 days after VCA, scale bar: 50 µm. (C and D) Representative figures for H&E and immunohistochemistry (IHC) with CD8, CD4 and Foxp3 antibodies either with vehicle or β2‐agonist treatment for 7 days. (E) Numbers of CD4, CD8 and Foxp3 positive cells in grafts 7 days after VCA. Nine fields from three grafts per group. ns, not significant; error bar, standard error of the mean. (F) Compositions of CD4+/CD8+ central memory (CM) and effector memory (EM) T‐cell populations in transplanted grafts 7 days after VCA. Control, non‐vascularized grafts; n = 5. ns, not significant; error bar, standard error of the mean; scale bar: 400 µm. *p < .05 by Student's t test
FIGURE S4 Pathology findings of CD4 T‐cell infiltration 5 days after VCA. Representative IHC images of nine different transplanted grafts revealed CD4 T‐cell infiltration in the vehicle (A) and β2‐agonist (B) injected groups, scale bar: 400 µm
FIGURE S5 Pathology findings of CD8 T‐cell infiltration 5 days after VCA. Representative IHC images of nine different transplanted grafts revealed CD8 T‐cell infiltration in the vehicle (A) and β2‐agonist (B) injected groups, scale bar: 400 µm
FIGURE S6 Pathology findings of Foxp3 cell infiltration 5 days after VCA. Representative IHC images of nine different transplanted grafts revealed Foxp3 cell infiltration in the vehicle (A) and β2‐agonist (B) injected groups, scale bar: 400 µm
FIGURE S7 The composition of donor's and recipient's leukocytes in donor grafts and recipient blood 5 and 7 days after VCA. (A and B) Source of infiltrated T cells in transplanted grafts and recipient's blood between recipient (CD45‐1) and donor (CD45‐2). Control, non‐vascularized grafts; n ≥ 3. ns, not significant; error bar, standard error of the mean. *p < .05 by Student's t test
FIGURE S8 The correlation of T‐cell infiltration and numbers of Foxp3+ cell in transplanted grafts after VCA. (A) Correlation analysis between numbers of infiltrated CD4/8 T and Foxp3+ cells in transplanted grafts (n = 8) 5 days after vehicle injections. (B) Correlation analysis between numbers of infiltrated CD4/8 T and Foxp3+ cells in transplanted grafts (n = 8) 5 days after β2‐agonist injections. The composition of Treg (CD4+CD25+Foxp3+) population was analysed with fTac injections after VCA. (C) Representative figure for H&E and IHC with numbers of CD4, CD8 and Foxp3 cells 10 days after VCA. Five fields from three grafts. Error bar, standard error of the mean; scale bar: 400 µm. (D) The Treg population was analysed with recipient's spleen 30 days after VCA. Control, a mouse without VCA; n = 4. ns, not significant; error bar, standard error of the mean
FIGURE S9 Preconditioning in donors with β2‐AR agonist delays rejection responses through suppression of T‐cell trafficking in the grafts. (A) BALB/c donor mice were injected with β2‐agonist for 2 weeks before VCA (pre‐VCA), and then β2‐agonist treatment was stopped after the surgery in C57BL/6 AR KO recipients. Representative figures, scale bar: 400 µm. (B) Systemic compositions of CD4+/CD8+ CM and EM T‐cell populations 7 days after VCA. (C) Th1‐ and Treg‐cell populations in CD4+ T cells. n ≥ 4 mice. ns, not significant; error bar, standard error of the mean. *p < .05 by Student's t test. (D) Numbers of CD4 and CD8 positive cells in grafts 7 days after VCA. Over 10 fields from 3 mice/group. ns, not significant; error bar, standard error of the mean; scale bar: 400 µm. Data with empty circles (post‐VCA); historical data. *p < .05 by Student's t test
TABLE S1 Two‐way ANOVA analysis in the number of infiltrating CD4+ T and CD8+ T cells from day 5 to 7
TABLE S2 Two‐way ANOVA analysis in changes of cytokine levels from day 5 to 7
TABLE S3 Two‐way ANOVA analysis in changes of chemokine levels from day 5 to 7
Supporting Information
ACKNOWLEDGEMENTS
The authors would like to thank Drs. Michelle Appenheimer, Bonnie Hylander and Hemn Mohammadpour for their constructive criticism of the manuscript. This research was supported by R21AI136367‐01A1 from NIH NIAID, Roswell Park Alliance Foundation and Cancer Center support grant P30CA06156.
Kim M, Fisher DT, Bogner PN, et al. Manipulating adrenergic stress receptor signalling to enhance immunosuppression and prolong survival of vascularized composite tissue transplants. Clin Transl Med. 2022;12:e996. 10.1002/ctm2.996
Contributor Information
Minhyung Kim, Email: Minhyung.Kim@RoswellPark.org.
Elizabeth A. Repasky, Email: Elizabeth.Repasky@RoswellPark.org.
REFERENCES
- 1. Fleming ME, Bharmal H, Valerio I. Regenerative medicine applications in combat casualty care. Regen Med. 2014;9(2):179‐190. [DOI] [PubMed] [Google Scholar]
- 2. Fleming M, Waterman S, Dunne J, et al. Dismounted complex blast injuries: patterns of injuries and resource utilization associated with the multiple extremity amputee. J Surg Orthop Adv. 2012;21(1):32‐37. [PubMed] [Google Scholar]
- 3. Gawande A. Casualties of war–military care for the wounded from Iraq and Afghanistan. N Engl J Med. 2004;351(24):2471‐2475. [DOI] [PubMed] [Google Scholar]
- 4. Petit F, Minns AB, Dubernard JM, et al. Composite tissue allotransplantation and reconstructive surgery: first clinical applications. Ann Surg. 2003;237(1):19‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Siemionow M, Kulahci Y. Facial transplantation. Semin Plast Surg. 2007;21(4):259‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Devauchelle B, Badet L, Lengele B, et al. First human face allograft: early report. Lancet. 2006;368(9531):203‐209. [DOI] [PubMed] [Google Scholar]
- 7. Dubernard JM, Owen ER, Lanzetta M, et al. What is happening with hand transplants. Lancet. 2001;357(9269):1711‐1712. [DOI] [PubMed] [Google Scholar]
- 8. Iske J, Nian Y, Maenosono R, et al. Composite tissue allotransplantation: opportunities and challenges. Cell Mol Immunol. 2019;16(4):343‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gilbert R. Transplant is successful with a cadaver forearm. Med Trib Med News. 1964;5:20‐23. [Google Scholar]
- 10. Gupta A, Kumer S, Kaplan B. Novel immunosuppressive strategies for composite tissue allografts. Curr Opin Organ Transplant. 2014;19(6):552‐557. [DOI] [PubMed] [Google Scholar]
- 11. Dubernard JM, Owen E, Herzberg G, et al. Human hand allograft: report on first 6 months. Lancet. 1999;353(9161):1315‐1320. [DOI] [PubMed] [Google Scholar]
- 12. Jones JW, Gruber SA, Barker JH, et al. Successful hand transplantation. One‐year follow‐up. Louisville Hand Transplant Team. N Engl J Med. 2000;343(7):468‐473. [DOI] [PubMed] [Google Scholar]
- 13. Kueckelhaus M, Fischer S, Seyda M, et al. Vascularized composite allotransplantation: current standards and novel approaches to prevent acute rejection and chronic allograft deterioration. Transpl Int. 2016;29(6):655‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Petruzzo P, Lanzetta M, Dubernard JM, et al. The international registry on hand and composite tissue transplantation. Transplantation. 2010;90(12):1590‐1594. [DOI] [PubMed] [Google Scholar]
- 15. Lucio MJ, Horta R. Hand transplantation—risks and benefits. J Hand Microsurg. 2021;13(4):207‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Basu A, Contreras AG, Datta D, et al. Overexpression of vascular endothelial growth factor and the development of post‐transplantation cancer. Cancer Res. 2008;68(14):5689‐5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gallagher MP, Kelly PJ, Jardine M, et al. Long‐term cancer risk of immunosuppressive regimens after kidney transplantation. J Am Soc Nephrol. 2010;21(5):852‐858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anonymous . World's first face transplant recipient Isabelle Dinoire dies of cancer. The Daily Telegraph. September 6, 2016. [Google Scholar]
- 19. Chavan SS, Ma P, Chiu IM. Neuro‐immune interactions in inflammation and host defense: implications for transplantation. Am J Transplant. 2018;18(3):556‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sundman E, Olofsson PS. Neural control of the immune system. Adv Physiol Educ. 2014;38(2):135‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Forsythe P. The nervous system as a critical regulator of immune responses underlying allergy. Curr Pharm Des. 2012;18(16):2290‐2304. [DOI] [PubMed] [Google Scholar]
- 22. Madva EN, Granstein RD. Nerve‐derived transmitters including peptides influence cutaneous immunology. Brain Behav Immun. 2013;34:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Padgett DA, Glaser R. How stress influences the immune response. Trends Immunol. 2003;24(8):444‐448. [DOI] [PubMed] [Google Scholar]
- 24. Glaser R, Kiecolt‐Glaser JK. Stress‐induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5(3):243‐251. [DOI] [PubMed] [Google Scholar]
- 25. Smith SM, Vale WW. The role of the hypothalamic‐pituitary‐adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8(4):383‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Herman JP, McKlveen JM, Ghosal S, et al. Regulation of the hypothalamic‐pituitary‐adrenocortical stress response. Compr Physiol. 2016;6(2):603‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987‐2007). Brain Behav Immun. 2007;21(6):736‐745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kenney MJ, Ganta CK. Autonomic nervous system and immune system interactions. Compr Physiol. 2014;4(3):1177‐1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sharma D, Farrar JD. Adrenergic regulation of immune cell function and inflammation. Semin Immunopathol. 2020;42(6):709‐717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eng JW, Reed CB, Kokolus KM, et al. Housing temperature‐induced stress drives therapeutic resistance in murine tumour models through β2‐adrenergic receptor activation. Nat Commun. 2015;6:6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bucsek MJ, Qiao G, MacDonald CR, et al. Beta‐adrenergic signaling in mice housed at standard temperatures suppresses an effector phenotype in CD8(+) T cells and undermines checkpoint inhibitor therapy. Cancer Res. 2017;77(20):5639‐5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Qiao G, Bucsek MJ, Winder NM, et al. Beta‐Adrenergic signaling blocks murine CD8(+) T‐cell metabolic reprogramming during activation: a mechanism for immunosuppression by adrenergic stress. Cancer Immunol Immunother. 2019;68(1):11‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qiao G, Chen M, Bucsek MJ, et al. Adrenergic signaling: a targetable checkpoint limiting development of the antitumor immune response. Front Immunol. 2018;9:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mohammadpour H, MacDonald CR, Qiao G, et al. β2 adrenergic receptor‐mediated signaling regulates the immunosuppressive potential of myeloid‐derived suppressor cells. J Clin Invest. 2019;129(12):5537‐5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leigh ND, Kokolus KM, O'Neill RE, et al. Housing temperature‐induced stress is suppressing murine graft‐versus‐host disease through β2‐adrenergic receptor signaling. J Immunol. 2015;195(10):5045‐5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mohammadpour H, Sarow JL, MacDonald CR, et al. β2‐Adrenergic receptor activation on donor cells ameliorates acute GvHD. JCI Insight. 2020;5(12):e137788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim M, Fisher DT, Powers CA, et al. Improved cuff technique and intraoperative detection of vascular complications for hind limb transplantation in mice. Transplant Direct. 2018;4(2):e345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang Y, Thatcher SE, Cassis LA. Measuring blood pressure using a noninvasive tail cuff method in mice. Methods Mol Biol. 2017;1614:69‐73. [DOI] [PubMed] [Google Scholar]
- 39. Regan RD, Fenyk‐Melody JE, Tran SM, et al. Comparison of submental blood collection with the retroorbital and submandibular methods in mice (Mus musculus). J Am Assoc Lab Anim Sci. 2016;55(5):570‐576. [PMC free article] [PubMed] [Google Scholar]
- 40. Fisher DT, Chen Q, Skitzki JJ, et al. IL‐6 trans‐signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells. J Clin Invest. 2011;121(10):3846‐3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ku AW, Muhitch JB, Powers CA, et al. Tumor‐induced MDSC act via remote control to inhibit L‐selectin‐dependent adaptive immunity in lymph nodes. eLife. 2016;5:e17375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cendales LC, Kanitakis J, Schneeberger S, et al. The Banff 2007 working classification of skin‐containing composite tissue allograft pathology. Am J Transplant. 2008;8(7):1396‐1400. [DOI] [PubMed] [Google Scholar]
- 43. Vasilic D, Alloway RR, Barker JH, et al. Risk assessment of immunosuppressive therapy in facial transplantation. Plast Reconstr Surg. 2007;120(3):657‐668. [DOI] [PubMed] [Google Scholar]
- 44. Zhao X, Ho D, Gao S, et al. Arterial pressure monitoring in mice. Curr Protoc Mouse Biol. 2011:1105‐1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007;45(2):27‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Panes J, Perry M, Granger DN. Leukocyte‐endothelial cell adhesion: avenues for therapeutic intervention. Br J Pharmacol. 1999;126(3):537‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hughes CE, Nibbs RJB. A guide to chemokines and their receptors. FEBS J. 2018;285(16):2944‐2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol. 2002;283(1):R7‐28. [DOI] [PubMed] [Google Scholar]
- 49. Schneeberger S, Landin L, Jableki J, et al. Achievements and challenges in composite tissue allotransplantation. Transpl Int. 2011;24(8):760‐769. [DOI] [PubMed] [Google Scholar]
- 50. Howsare M, Jones CM, Ramirez AM. Immunosuppression maintenance in vascularized composite allotransplantation: what is just right?. Curr Opin Organ Transplant. 2017;22(5):463‐469. [DOI] [PubMed] [Google Scholar]
- 51. Cavadas PC, Ibanez J, Thione A, et al. Bilateral trans‐humeral arm transplantation: result at 2 years. Am J Transplant. 2011;11(5):1085‐1090. [DOI] [PubMed] [Google Scholar]
- 52. Kaufman CL, Ouseph R, Blair B, et al. Graft vasculopathy in clinical hand transplantation. Am J Transplant. 2012;12(4):1004‐1016. [DOI] [PubMed] [Google Scholar]
- 53. Barisione G, Baroffio M, Crimi E, et al. Beta‐adrenergic agonists. Pharmaceuticals (Basel). 2010;3(4):1016‐1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Totterman KJ, Huhti L, Sutinen E, et al. Tolerability to high doses of formoterol and terbutaline via Turbuhaler for 3 days in stable asthmatic patients. Eur Respir J. 1998;12(3):573‐579. [DOI] [PubMed] [Google Scholar]
- 55. Speakman JR. Measuring energy metabolism in the mouse – theoretical, practical, and analytical considerations. Front Physiol. 2013;4:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362(9):779‐789. [DOI] [PubMed] [Google Scholar]
- 57. Qiao G, Chen M, Mohammadpour H, et al. Chronic adrenergic stress contributes to metabolic dysfunction and an exhausted phenotype in T cells in the tumor microenvironment. Cancer Immunol Res. 2021;9(6):651‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3(3):199‐210. [DOI] [PubMed] [Google Scholar]
- 59. Scalea JR, Lee YS, Davila E, et al. Myeloid‐derived suppressor cells and their potential application in transplantation. Transplantation. 2018;102(3):359‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Iglesias‐Escudero M, Sansegundo‐Arribas D, Riquelme P, et al. Myeloid‐derived suppressor cells in kidney transplant recipients and the effect of maintenance immunotherapy. Front Immunol. 2020;11:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Iglesias‐Escudero M, Segundo DS, Merino‐Fernandez D, et al. Myeloid‐derived suppressor cells are increased in lung transplant recipients and regulated by immunosuppressive therapy. Front Immunol. 2021;12:788851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cosentino G, Soprana E, Thienes CP, et al. IL‐13 down‐regulates CD14 expression and TNF‐alpha secretion in normal human monocytes. J Immunol. 1995;155(6):3145‐3151. [PubMed] [Google Scholar]
- 63. Albanesi C, Fairchild HR, Madonna S, et al. IL‐4 and IL‐13 negatively regulate TNF‐alpha‐ and IFN‐gamma‐induced β‐defensin expression through STAT‐6, suppressor of cytokine signaling (SOCS)‐1, and SOCS‐3. J Immunol. 2007;179(2):984‐992. [DOI] [PubMed] [Google Scholar]
- 64. Issekutz AC, Meager A, Otterness I, et al. The role of tumour necrosis factor‐alpha and IL‐1 in polymorphonuclear leucocyte and T lymphocyte recruitment to joint inflammation in adjuvant arthritis. Clin Exp Immunol. 1994;97(1):26‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Turner MD, Nedjai B, Hurst T, et al. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta. 2014;1843(11):2563‐2582. [DOI] [PubMed] [Google Scholar]
- 66. McLaughlin F, Hayes BP, Horgan CM, et al. Tumor necrosis factor (TNF)‐alpha and interleukin (IL)‐1β down‐regulate intercellular adhesion molecule (ICAM)‐2 expression on the endothelium. Cell Adhes Commun. 1998;6(5):381‐400. [DOI] [PubMed] [Google Scholar]
- 67. Lehmann JC, Jablonski‐Westrich D, Haubold U, et al. Overlapping and selective roles of endothelial intercellular adhesion molecule‐1 (ICAM‐1) and ICAM‐2 in lymphocyte trafficking. J Immunol. 2003;171(5):2588‐2593. [DOI] [PubMed] [Google Scholar]
- 68. Lyck R, Enzmann G. The physiological roles of ICAM‐1 and ICAM‐2 in neutrophil migration into tissues. Curr Opin Hematol. 2015;22(1):53‐59. [DOI] [PubMed] [Google Scholar]
- 69. Lemmens S, Kusters L, Bronckaers A, et al. The β2‐adrenoceptor agonist terbutaline stimulates angiogenesis via Akt and ERK signaling. J Cell Physiol. 2017;232(2):298‐308. [DOI] [PubMed] [Google Scholar]
- 70. Huang MT, Mason JC, Birdsey GM, et al. Endothelial intercellular adhesion molecule (ICAM)‐2 regulates angiogenesis. Blood. 2005;106(5):1636‐1643. [DOI] [PubMed] [Google Scholar]
- 71. Billington CK, Penn RB, Hall IP. β2 Agonists. Handb Exp Pharmacol. 2017;237:23‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vang T, Torgersen KM, Sundvold V, et al. Activation of the COOH‐terminal Src kinase (Csk) by cAMP‐dependent protein kinase inhibits signaling through the T cell receptor. J Exp Med. 2001;193(4):497‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kuo YR, Chen CC, Shih HS, et al. Prolongation of composite tissue allotransplant survival by treatment with bone marrow mesenchymal stem cells is correlated with T‐cell regulation in a swine hind‐limb model. Plast Reconstr Surg. 2011;127(2):569‐579. [DOI] [PubMed] [Google Scholar]
- 74. Sachs DH, Kawai T, Sykes M. Induction of tolerance through mixed chimerism induction of tolerance through mixed chimerism. Cold Spring Harb Perspect Med. 2014;4(1):a015529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Oura T, Hotta K, Cosimi AB, et al. Transient mixed chimerism for allograft tolerance. Chimerism. 2015;6(1‐2):21‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Schnuelle P, Lorenz D, Mueller A, et al. Donor catecholamine use reduces acute allograft rejection and improves graft survival after cadaveric renal transplantation. Kidney Int. 1999;56(2):738‐746. [DOI] [PubMed] [Google Scholar]
- 77. Schnuelle P, Yard BA, Braun C, et al. Impact of donor dopamine on immediate graft function after kidney transplantation. Am J Transplant. 2004;4(3):419‐426. [DOI] [PubMed] [Google Scholar]
- 78. Leal E, Liu C, Zhao Z, et al. Isolation of a divergent strain of bovine parainfluenza virus type 3 (BPIV3) infecting cattle in China. Viruses. 2019;11(6):489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Scanzano A, Cosentino M. Adrenergic regulation of innate immunity: a review. Front Pharmacol. 2015;6:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wieduwild E, Girard‐Madoux MJ, Quatrini L, et al. β2‐adrenergic signals downregulate the innate immune response and reduce host resistance to viral infection. J Exp Med. 2020;217(4):e20190554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Gating strategies for flow cytometry
FIGURE S2 A schematic illustration of our vascularized composite tissue allotransplantation (VCA) model and the safety of a selective β2‐adrenergic receptor (AR) agonist drug, terbutaline. (A) BALB/c and C57BL/6 strains were used as donors and recipients respectively. En bloc tissue composed of skin, subcutaneous fat, muscle, vessels and femur was transplanted to a recipient's cervical area. (B) Systolic and diastolic blood pressures (BP) with heart rates (HRs) were measured after everyday injection of with either vehicle (V; PBS) or β2‐agonist (β; terbutaline; 2 mg/day). Mice were acclimated to the BP and HR measuring procedures for 10 days before recording data. Representative data between two different experiments, n = 5. (C) Concentrations of tacrolimus in plasma were analysed 14 days after subcutaneous injections (24 h after the last injection) with either a half dose of tacrolimus (hTac; 2 mg/kg/day) or a full dose of tacrolimus (fTac; 4 mg/kg/day). Representative data between two different experiments, n = 5. ns, not significant; error bar, standard error of the mean. *p < .05 by Student's t test
FIGURE S3 Pathologic findings 5 and 7 days after VCA with either vehicle or β2‐agonist injections. (A and B) Representative haematoxylin and eosin (H&E) images revealed epithelial dyskeratosis (arrowhead) and apoptosis (arrow) in the vehicle‐injected group (A; rejection grade 3) not in the β2‐agonist‐injected group (B; rejection grade 2) 5 days after VCA, scale bar: 50 µm. (C and D) Representative figures for H&E and immunohistochemistry (IHC) with CD8, CD4 and Foxp3 antibodies either with vehicle or β2‐agonist treatment for 7 days. (E) Numbers of CD4, CD8 and Foxp3 positive cells in grafts 7 days after VCA. Nine fields from three grafts per group. ns, not significant; error bar, standard error of the mean. (F) Compositions of CD4+/CD8+ central memory (CM) and effector memory (EM) T‐cell populations in transplanted grafts 7 days after VCA. Control, non‐vascularized grafts; n = 5. ns, not significant; error bar, standard error of the mean; scale bar: 400 µm. *p < .05 by Student's t test
FIGURE S4 Pathology findings of CD4 T‐cell infiltration 5 days after VCA. Representative IHC images of nine different transplanted grafts revealed CD4 T‐cell infiltration in the vehicle (A) and β2‐agonist (B) injected groups, scale bar: 400 µm
FIGURE S5 Pathology findings of CD8 T‐cell infiltration 5 days after VCA. Representative IHC images of nine different transplanted grafts revealed CD8 T‐cell infiltration in the vehicle (A) and β2‐agonist (B) injected groups, scale bar: 400 µm
FIGURE S6 Pathology findings of Foxp3 cell infiltration 5 days after VCA. Representative IHC images of nine different transplanted grafts revealed Foxp3 cell infiltration in the vehicle (A) and β2‐agonist (B) injected groups, scale bar: 400 µm
FIGURE S7 The composition of donor's and recipient's leukocytes in donor grafts and recipient blood 5 and 7 days after VCA. (A and B) Source of infiltrated T cells in transplanted grafts and recipient's blood between recipient (CD45‐1) and donor (CD45‐2). Control, non‐vascularized grafts; n ≥ 3. ns, not significant; error bar, standard error of the mean. *p < .05 by Student's t test
FIGURE S8 The correlation of T‐cell infiltration and numbers of Foxp3+ cell in transplanted grafts after VCA. (A) Correlation analysis between numbers of infiltrated CD4/8 T and Foxp3+ cells in transplanted grafts (n = 8) 5 days after vehicle injections. (B) Correlation analysis between numbers of infiltrated CD4/8 T and Foxp3+ cells in transplanted grafts (n = 8) 5 days after β2‐agonist injections. The composition of Treg (CD4+CD25+Foxp3+) population was analysed with fTac injections after VCA. (C) Representative figure for H&E and IHC with numbers of CD4, CD8 and Foxp3 cells 10 days after VCA. Five fields from three grafts. Error bar, standard error of the mean; scale bar: 400 µm. (D) The Treg population was analysed with recipient's spleen 30 days after VCA. Control, a mouse without VCA; n = 4. ns, not significant; error bar, standard error of the mean
FIGURE S9 Preconditioning in donors with β2‐AR agonist delays rejection responses through suppression of T‐cell trafficking in the grafts. (A) BALB/c donor mice were injected with β2‐agonist for 2 weeks before VCA (pre‐VCA), and then β2‐agonist treatment was stopped after the surgery in C57BL/6 AR KO recipients. Representative figures, scale bar: 400 µm. (B) Systemic compositions of CD4+/CD8+ CM and EM T‐cell populations 7 days after VCA. (C) Th1‐ and Treg‐cell populations in CD4+ T cells. n ≥ 4 mice. ns, not significant; error bar, standard error of the mean. *p < .05 by Student's t test. (D) Numbers of CD4 and CD8 positive cells in grafts 7 days after VCA. Over 10 fields from 3 mice/group. ns, not significant; error bar, standard error of the mean; scale bar: 400 µm. Data with empty circles (post‐VCA); historical data. *p < .05 by Student's t test
TABLE S1 Two‐way ANOVA analysis in the number of infiltrating CD4+ T and CD8+ T cells from day 5 to 7
TABLE S2 Two‐way ANOVA analysis in changes of cytokine levels from day 5 to 7
TABLE S3 Two‐way ANOVA analysis in changes of chemokine levels from day 5 to 7
Supporting Information


