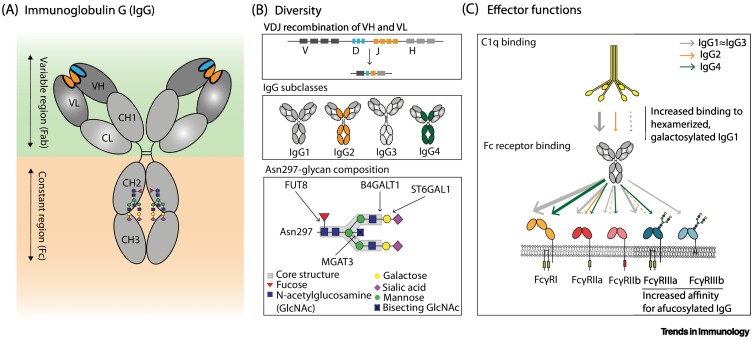
Figure 1.
Diversity and effector functions of human IgG.
(A) Schematic representation of IgG with variable (Fab) and constant (Fc) regions in the green and orange panels, respectively. The localization of the conserved Asn297 glycan in the Fc domain (CH2) is indicated [2]. (B) Antibody diversity arises during B cell development when rearrangement of variable, diversity, and joining gene regions [V(D)J] results in a diverse repertoire of antigen-specificities (upper panel) [2]. Upon B cell activation, B cells undergo isotype switching allowing them to produce antibodies of the IgG class, which is further subdivided into IgG1, IgG2, IgG3 and IgG4 subclasses (middle panel) [2]. The variable composition of the Asn297 glycan also contributes to IgG diversity, and different glycosyltransferases are responsible for enzymatic addition of residues to the core structure (GlcNAc4Man3) [30]. These modifications include the addition of galactose (B4GALT1), sialic acid (ST6GALT), bisecting GlcNAc (MGAT3), and core fucose (FUT8) (lower panel) [30]. (C) The diverse repertoire of IgG shapes effector functions, including the interaction of IgG-Fc with C1q, to activate the complement system, and FcγRs, to elicit antibody-dependent cellular cytotoxicity and phagocytosis [antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP)] [2]. The affinities of the different IgG subclasses for effector molecules are indicated by the individual color and thickness of the arrows [5]. Only the Asn162 glycan present in FcγRIIIa is shown as a biantennary glycan, although all FcγRs contain multiple N-linked glycans and also some of high-mannose structures. Abbreviations: CH1–3, immunoglobulin heavy-chain constant regions 1–3; CL, light-chain constant region; VH, heavy-chain variable region; VL, light-chain variable region.