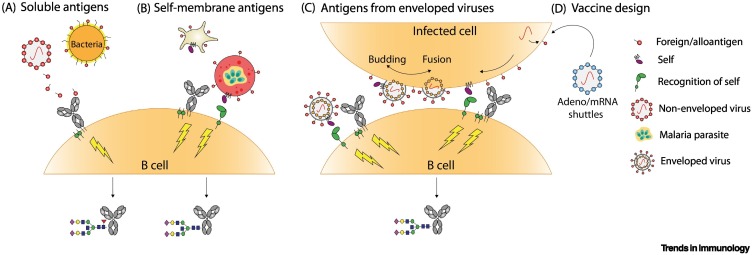
Figure 3.
Key Figure. Hypothetical model illustrating the possible role of antigen context-induced alteration of immune signaling and the production of afucosylated IgG in humans.
(A) Soluble antigens, derived from non-enveloped viruses or bacteria, signal through the B cell receptor (BCR) resulting in B cell activation and the production of fucosylated antigen-specific IgG [79., 80., 81., 82.]. (B) For alloantigens on platelets and red blood cells (RBCs) (not shown), and Plasmodium falciparum proteins expressed on infected RBCs, we propose that antigen recognition by the BCR is accompanied by a so far unknown receptor–ligand pair that provides a signal for 'self'. Signaling through these complexes is required for the induction of afucosylated IgG [14,18,20,22]. (C) Similarly, responses towards enveloped viral infections resemble responses described in (B) for alloantigens and P. falciparum proteins, as these antigens are also self-membrane-bound [27]. Self-recognition might either occur from the virion (left) if segments of the host cell membrane are included in the viral envelope, or (right) from virus-infected cells as the viral envelope fuses with the host membrane [83], both resulting in the production of afucosylated antigen-specific IgG [26., 27., 28.]. (D) The potency of afucosylated IgG might be harnessed in vaccine design by mimicking natural antigen display as depicted in (B) and (C). Using mRNA templates or viral shuttles for transcription in host cells, foreign antigens might be expressed in the context of a self-membrane which could induce afucosylated IgG [64]. Abbreviation: adeno, adenovirus.