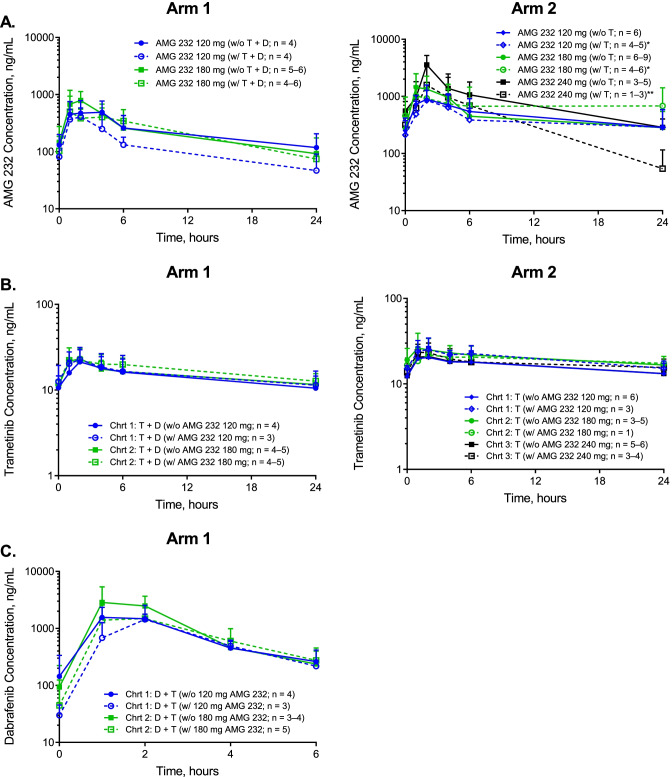
Fig. 2.
Plasma pharmacokinetics of AMG 232, trametinib, and dabrafenib at steady-state for combinations of AMG 232 with trametinib and dabrafenib (Arm 1) or trametinib (Arm 2) given orally in adult subjects with MM. In Arms 1 and 2, AMG 232 was administered QD on days 1–7 of each cycle with a 14-day treatment-free period on days 8–21. In addition, trametinib was dosed continuously QD starting on day 8 of cycle 1. In Arm 1 only, dabrafenib was dosed continuously BID starting on day 8 of cycle 1. A. Mean (+SD) plasma concentration-time profiles of AMG 232 following AMG 232 dosing on cycle 1, day 7, when administered alone or on cycle 2, day 7, when co-administered with trametinib and dabrafenib [Arm 1] or trametinib [Arm 2]. B. Mean (+SD) plasma concentration-time profiles of trametinib following dosing of trametinib and dabrafenib [Arm 1] or trametinib alone [Arm 2] on cycle 1, day 21, without co-administration of AMG 232 (at the end of the 14-day AMG 232 treatment-free period) or on cycle 2, day 7, when co-administered with AMG 232. C. Mean (+SD) plasma concentration-time profiles of dabrafenib following dosing of dabrafenib and trametinib in Arm 1 on cycle 1, day 21, without co-administration of AMG 232 (at the end of the 14-day AMG 232 treatment-free period) or on cycle 2, day 7, when co-administered with AMG 232. Abbreviations: BID, twice a day; Chrt, cohort; D, dabrafenib; QD, once daily; SD, standard deviation; T, trametinib