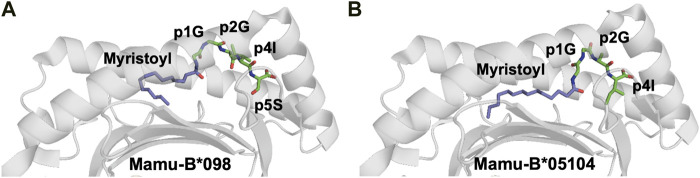
FIGURE 5.
The myristoyl moiety in myristoylated peptides plays an essential role in both MHC-I binding and adequate presentation of the epitope (A). The crystal structure of the rhesus macaque MHC-I molecule Mamu-B*098 in complex with the myristoylated 5-mer lipopeptide derived from SIV Nef protein (4ZFZ.pdb), reveals the dual role of the PTM moiety in both adequate binding to the MHC-I and peptide presentation (Morita and Sugita 2016). (B). The crystal structure of the rhesus macaque MHC-I molecule Mamu-B*05,104 in complex with a N-myristoylated 4-mer lipopeptide derived from the SIV nef protein (6IWG.pdb) shows how the myristoyl moiety occupies the cleft differently (Yamamoto et al., 2019)