FIGURE 6.
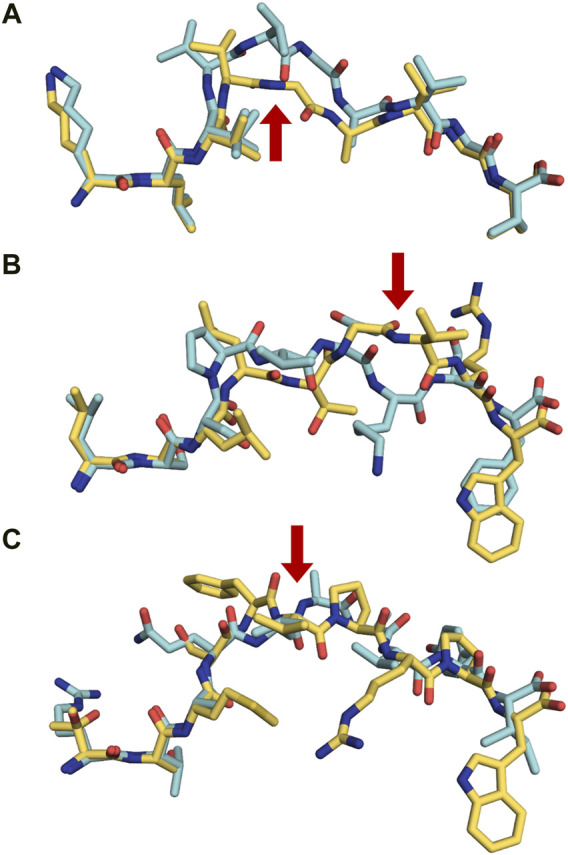
Proteasomally spliced peptides bind in a canonical mode to MHC molecules (A) Comparison of the conformations of the original KRAS5-14 peptide (6O53. pdb, cyan) and its cis-spliced variant KRAS5-6/8–14 (6O4Y.pdb, yellow) both in complex with HLA-A2*01 demonstrates that the splicing results in the formation of a neoepitope through the removal of a central conformational bulge following splicing (Mishto et al., 2019). The red arrow shows the junction between spliced fragments. (B) Although sequentially unrelated, a comparison of the linear nonameric self-peptide 94-LSSPVTKSF-102 derived from immunoglobulin kappa (2RFX.pdb, cyan) (Chessman et al., 2008) and the nonameric cis-peptide LALLTGVRW (6D2T.pdb, yellow) reveals that i) they make use of similar anchor residues (p2A/p2S and pF9/pW9) in order to bind to HLA-B*57:01 (Faridi et al., 2018; Mishto et al., 2019), and ii) that the junction between spliced fragments is protruding towards the solvent, readily available for interactions with TCRs (red arrow) (C). A similar comparison is presented here with two unrelated decameric peptides. The junction between spliced fragments in the trans-spliced epitope TSMSFVPRPW (6D29. pdb, yellow) (Faridi et al., 2018) is also available for interactions with TCRs. The HLA-B*57:01-restricted decameric peptide from small nuclear protein SmD3 54-RVAQLEQVYI-63 (3VRI.pdb) is presented as a reference for a conventional epitope (Illing et al., 2018). The red arrow shows the junction between spliced fragments.
