FIGURE 7.
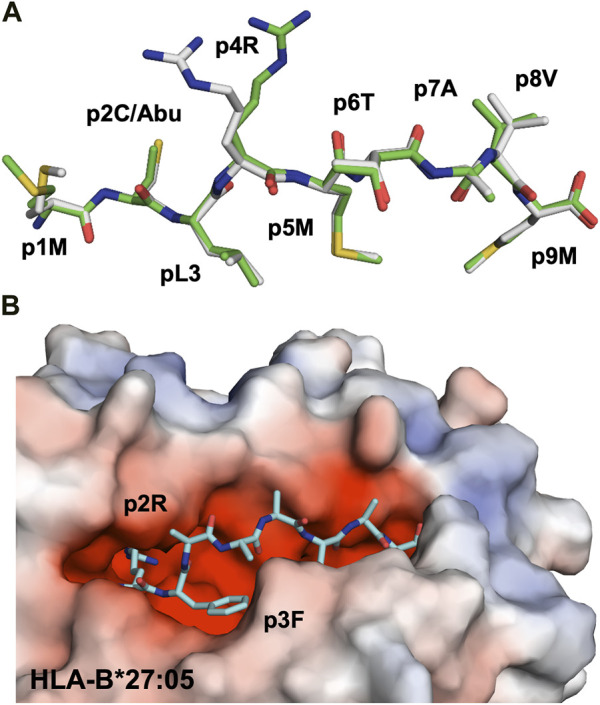
Unnatural amino acids can be easily used to assess the importance of peptide residues for pMHC stability and TCR recognition (A) The unnatural aa α-aminobutyrate was used to replace a cysteine residue, abolishing sulfur–π interactions with this peptide residue and the H-2Db residue Y45 in H-2Db/Trh4, demonstrating the key role of this type of interaction for the efficient binding of this TEIPP peptide to H-2Db. Comparison of the crystal structures of H-2Db in complex with Trh4 (5E8N.pdb) or Thr4-p2ABU (5E8O.pdb) demonstrated that the conformation of the backbone of the altered peptide (green) is identical to wild-type Trh4 (grey) despite significant different overall pMHC stability (Hafstrand et al., 2016) (B) Replacement of two anchor residues in the peptide bound to HLA-B*27:05 (Hülsmeyer et al., 2004) with unnatural amino acids (1JGE.pdb) resulted in the destabilization of MHC/peptide complex and decreased immunogenicity (Jones et al., 2006).
