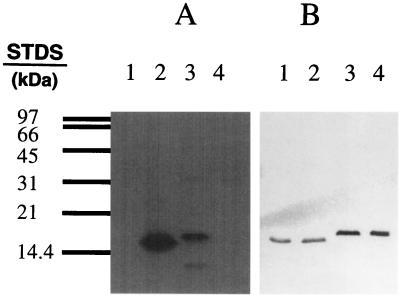
FIG. 5.
Site-directed mutagenesis suggests Slr1856 and Slr1859 are phosphorylated by Slr1861 on serine residues 54 and 57, respectively. A mutationally-altered form of Slr1856 in which Ser-54 was replaced with alanine, Slr1856 (Ser-54/Ala), and a mutationally altered form of Slr1859 in which Ser-57 was replaced by alanine (Slr1859) (Ser-57/Ala), were prepared and expressed as described in Materials and Methods. (A) Aliquots, containing 20 μg of protein each, from E. coli cells expressing either Slr1856 (Ser-54/Ala) (lane 1), Slr1856 (lane 2), Slr1859 (lane 3), or Slr1859 (Ser-57/Ala) (lane 4) were mixed with 20 μg of lysate from cells expressing Slr1861 in the presence of [γ-32P]ATP and analyzed by SDS-PAGE as described in Materials and Methods. Shown is the autoradiogram of the reaction mixture following SDS-PAGE. (B) Results of a Western blot, performed as described in Materials and Methods with the anti-Xpress antibody, of a duplicate gel containing 20 μg each of protein from lysates from E. coli expressing Slr1856 (Ser-54/Ala) (lane 1), Slr1856 (lane 2), Slr1859 (lane 3), and Slr1859 (Ser-57/Ala) (lane 4). STDs, standards.