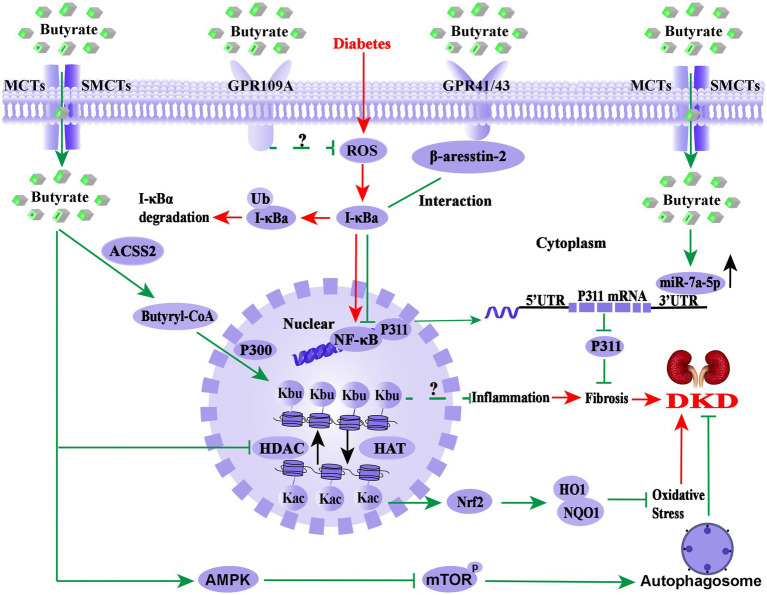
Figure 2.
Overview of the molecular mechanism of butyrate in the prevention and treatment of DKD. The pathological process of DKD involves persistent HG-induced oxidative stress, immune system disorders, and inflammation (red arrows). Endogenous or exogenous butyrate (green arrows) inhibits the activity of HDAC, opens the structure of chromatin, and facilitates the expression of the Nrf2 gene, which may enter the nucleus and upregulate the downstream targets HO1 and NQO1 and then inhibits oxidative stress and inflammation in DKD. Meanwhile, GPR43 and GPR109A are important receptors of butyrate for renal protection, and the interaction between β-arrestin-2 and I-κBα is induced by butyrate via GPR43, suggesting that butyrate-mediated GPR43-β-arrestin-2 signaling may be a novel and promising target for DKD (green arrows). Moreover, it has been found that butyrate reverses HG-induced the downregulation of miR-7a-5p and inhibits the expression of P311, followed by the inhabitation of the kidney fibrosis of DKD (green arrows) and activated autophagy via the AMPK/mTOR pathway to delay the DKD progression. Notably, butyl-CoA, a metabolite of butyrate, is the substrate of histone butyrylation modification, irrespective of whether butyrate or sodium butyrate improves DKD renal injury through histone butyrylation pathway or the cross-talk of the histone post-translational modifications has not been reported. Nrf2, Nuclear factor erythroid 2-related factor 2; HO1, heme oxygenase 1; NQO1, NAD(P)H dehydrogenase quinone 1; HDAC, histone deacetylase; HAT, histone acetyltransferase; UTR, untranslated region; NF-κB, nuclear factor kappa B; Kbu, histone lysine butyrylation; Kac, histone lysine acetylation; ACSS2, acetyl-CoA synthetase 2; p300, a histone acetylation transferase that mediates butyrylation; P311, an RNA-binding protein, which could stimulate fibrosis; AMPK, AMP-activated protein kinase; mTOR, mammalian target of rapamycin.