Figure 2.
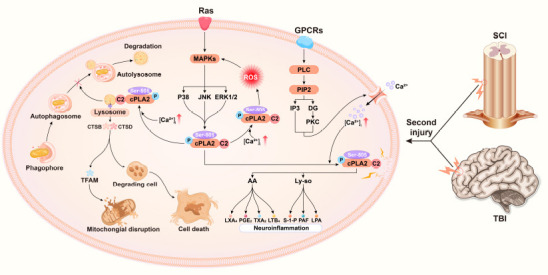
Graphical presentation of the mechanism of cPLA2 in traumatic brain and spinal cord injuries.
The extracellular signal molecule binds to its corresponding G-protein-coupled receptor to activate PLC. PLC cleaves the PIP2 in the cell membrane into DAG and IP3 and then activates PKC to cause a cascade reaction, which opens the calcium channel of the cell membrane and increases the intracellular calcium ion concentration. In addition, a second injury promotes cPLA2 phosphorylation through the ras-MAPK signaling pathway. The increased intracellular calcium promotes the binding of the C2 domain of cPLA2 to membrane phospholipids. Phosphorylated cPLA2 hydrolyses membrane phospholipids, producing downstream products (AA, Ly-so, and their subsequent metabolites) that trigger neuroinflammation. Phosphorylated cPLA2 simultaneously binds to the lysosomal membrane, resulting in increased permeability of the lysosomal membrane. Lysosomal enzymes (CTSB, CTSD) exosmose to degrade TFAM and impair mitochondrial function. Exosmotic lysosomal enzymes break down digestive cells and cause neuronal cell death. Damaged lysosomal enzymes prevent the binding of lysosomal enzymes to autophagosomes and thus hinder the progress of autophagy. [Ca2+]i: Intracellular calcium concentration; AA: arachidonic acid; CTSB: cathepsin B; CTSD: cathepsin D; DAG: diacylglycerol; IP3: inositol triphosphate; LPA: lysophosphatidic acid; LTB4: leukotriene B4; LXA4: lipoxin A4; Ly-so: lysophospholipids; MAPK: mitogen-activated protein kinase; PAF: platelet-activating factor; PGE2: prostaglandin E2; PIP2: phosphati-dylinositol-4,5-bisphosphate; PKC: protein kinase C; PLC: phospholipase C; S-1-P: sphingosine 1 phosphate; TFAM: transcription factor A; TXA2: thromboxane 2.
