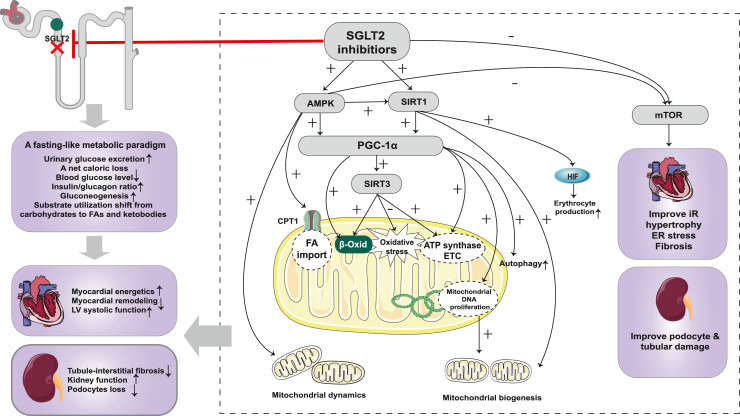
Figure 4.
Cardiorenal protection of SGLT2 inhibitor based on metabolic reprogramming. SGLT2 inhibition leads to marked glycosuria and a net caloric loss, which mimics a fasting-like metabolic response. The energy substrates shift from carbohydrates to FAs and ketone bodies, which restore ATP levels to exert cardiorenal protective effects. SGLT2 inhibitors induce a nutrient deprivation transcriptional paradigm via acting on nutrient-sensing pathways. SGLT2 inhibitors activate the AMPK/SIRT1/PGC-1α pathway, enhancing FAO and ketogenesis, which improve the energy state of the heart and kidney. In addition, the activated AMPK/SIRT1/PGC-1α pathway exerts other protective effects, including improving mitochondrial biogenesis and dynamics, enhancing autophagy, and attenuating oxidative stress. SGLT2 inhibitors inhibit the mTOR pathway and thereby improve insulin resistance, cardiac hypertrophy, fibrosis, and oxidative stress. In addition, inhibition of mTORC1 prevents tubular and podocyte damage, partially via the effects of increased ketone bodies on mTORC1. Abbreviations: SGLT2: sodium-glucose co-transporter 2; FA: fatty acids; LV: left ventricular; mTOR: mechanistic target of rapamycin; mTORC1: mechanistic target of rapamycin complex 1; SIRT1: silent information regulator 1; PGC-1α: peroxisome proliferator-activated receptor-gamma co-activator 1α; SIRT3: silent information regulator 3; HIF: hypoxia-inducible factor; CPT-1: carnitine palmitoyl transferase-1; β-Oxid: β-oxidation; ATP: adenosine triphosphate; ETC: electron transfer chain; DNA: deoxyribonucleic acid; IR: insulin resistance; ER: endoplasmic reticulum.