Table 1.
Data set of 5-benyl-4-thiazolinone derivatives along with their inhibitory activities.
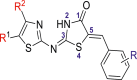 | |||||
|---|---|---|---|---|---|
| Sr No. | R1 | R2 | R | IC50 (μM) | pIC50 |
| 1 | CO2CH2CH3 | CH3 | 2-OH | 28.78 | 4.5409 |
| 2 | CO2CH2CH3 | CH3 | 3-OH | 43.57 | 4.3608 |
| 3 | CO2CH2CH3 | CH3 | 3-OCH3 | 73.11 | 4.1360 |
| 4 | CO2CH2CH3 | CH3 | 3-F | 64.38 | 4.1912 |
| 5 | CO2CH2CH3 | CH3 | 3-Cl | 78.72 | 4.1039 |
| 6 | CO2CH2CH3 | CH3 | 4-CO2H | 36.46 | 4.4381 |
| 7 | CO2CH2CH3 | CH3 | 4-CO2Me | 94.02 | 4.0267 |
| ∗8 | CO2CH2CH3 | CH3 | 4-NHAc | 44.11 | 4.3554 |
| 9 | CO2CH2CH3 | CH3 | 4-OH | 16.33 | 4.7870 |
| 10 | CO2CH2CH3 | CH3 | 2-OH-3-OCH3 | 19.21 | 4.7164 |
| 11 | CO2CH2CH3 | CH3 | 3-OCH3-4-OH | 13.06 | 4.8840 |
| 12 | CO2CH2CH3 | CH3 | 2,4-(OH)2 | 28.31 | 4.5480 |
| 13 | CO2CH2CH3 | CH3 | 3,4-(OH)2 | 27.11 | 4.5668 |
| 14 | CO2CH2CH3 | CH3 | 2-OH-3,5-di NO2 | 39.86 | 4.3994 |
| ∗15 | CO2H | CH3 | 3-OCH3-4-OH | 32.47 | 4.4885 |
| 16 | CO2CH3 | CH3 | 3-OCH3-4-OH | 30.39 | 4.5172 |
| 17 | CO2t-Bu | CH3 | 3-OCH3-4-OH | 27.53 | 4.5601 |
| 18 | CO2 CH3 | i-Pr | 4-OH | 19.21 | 4.7164 |
| ∗19 | CO2 CH3 | i-Pr | 2-OH-3-OCH3 | 28.90 | 4.5391 |
| 20 | CO2 CH3 | i-Pr | 3,4-(OH)2 | 21.81 | 4.6613 |
| 21 | CO2CH3 | i-Pr | 3-OCH3-4-OH | 22.98 | 4.6386 |
| 22 | CO2CH3 | i-Pr | 3,5-(OCH3)2-4-OH | 28.67 | 4.5425 |
| 23 | CO2CH3 | n-Pr | 3-OCH3-4-OH | 30.43 | 4.5166 |
| 24 | H | CH2CO2CH2CH3 | 3-NO2 | 46.00 | 4.3372 |
| 25 | H | CH2CO2CH2CH3 | 3-NH2 | 35.76 | 4.4466 |
| 26 | H | CH2CO2CH2CH3 | 4-CO2H | 34.71 | 4.4595 |
| ∗27 | H | CH2CO2CH2CH3 | 4-OH | 27.69 | 4.5576 |
| 28 | H | CH2CO2CH2CH3 | 2-OH-3-OCH3 | 33.90 | 4.4698 |
| 29 | H | CH2CO2CH2CH3 | 3-OH-4-OCH3 | 28.80 | 4.5406 |
| ∗30 | H | CH2CO2CH2CH3 | 3-OCH3-4-OH | 28.04 | 4.5522 |
| ∗31 | CH3 | CH2CO2CH2CH3 | 3-OCH3-4-OH | 20.30 | 4.6925 |
| 32 | Ac | CH3 | 3-NO2 | 70.54 | 4.1515 |
| 33 | Ac | CH3 | 3-OH | 19.09 | 4.7191 |
| ∗34 | Ac | CH3 | 4-CO2H | 31.75 | 4.4982 |
| 35 | Ac | CH3 | 4-OH | 20.53 | 4.6876 |
| 36 | Ac | CH3 | 3-OH-4-OCH3 | 18.28 | 4.7380 |
| 37 | Ac | CH3 | 3-OCH3-4-OH | 26.11 | 4.5831 |
| ∗38 | Ac | CH3 | 3,4-OCH2O | 42.28 | 4.3738 |
| ∗39 | Ac | CH3 | 3,4-(OH)2 | 25.30 | 4.5968 |
| 40 | NO2 | t-Bu | 2-CO2H | 33.02 | 4.4812 |
| 41 | NO2 | t-Bu | 2-OH | 35.36 | 4.4514 |
| 42 | NO2 | t-Bu | 3-NO2 | 48.24 | 4.3165 |
| 43 | NO2 | t-Bu | 3-OCH3 | 44.18 | 4.3547 |
| 44 | NO2 | t-Bu | 3-F | 32.08 | 4.4937 |
| 45 | NO2 | t-Bu | 4-NHAc | 75.78 | 4.1204 |
| 46 | NO2 | t-Bu | 4-OH | 38.82 | 4.4109 |
| ∗47 | NO2 | t-Bu | 2-OH-3-OCH3 | 55.03 | 4.2594 |
| ∗48 | NO2 | t-Bu | 3-OCH3-4-OH | 35.21 | 4.4533 |
superscript = test set.
