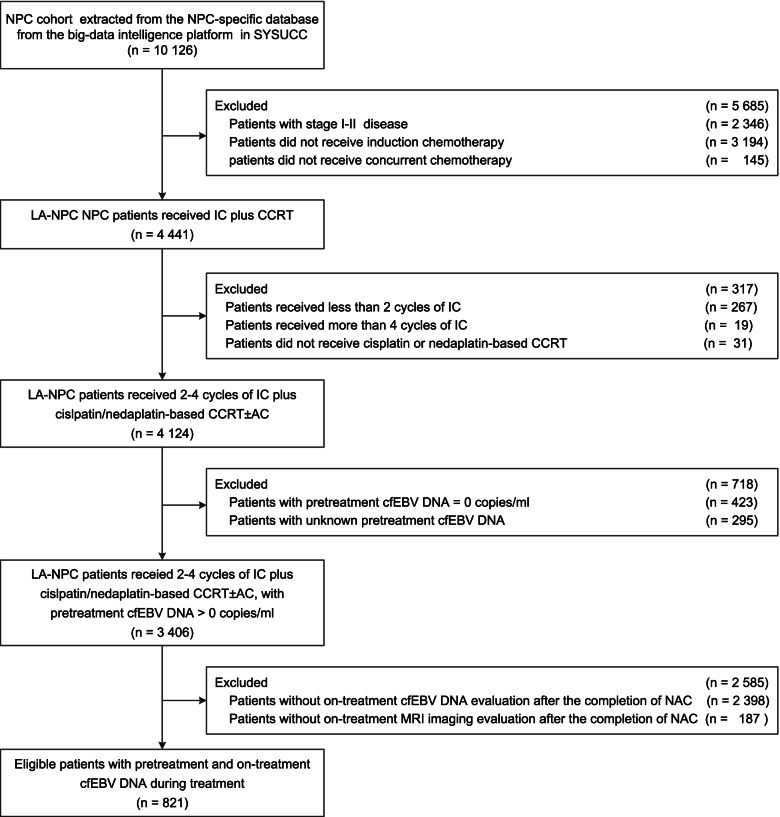
Fig. 1.
Flowchart showing the study design and patient selection process. The medical records of 10,126 patients with non-metastatic NPC were screened, and 821 patients with LA-NPC who received NAC plus concurrent CRT and had detectable pretreatment cfEBV DNA with on-treatment circulating cfEBV DNA surveillance were selected stepwise. Abbreviations: AC, adjuvant chemotherapy; CCRT, concurrent chemotherapy; cfEBV DNA, cell-free Epstein-Barr virus DNA; IC, induction chemotherapy; LA-NPC, locally advanced nasopharyngeal carcinoma; MRI, magnetic resonance imaging