Figure 3.
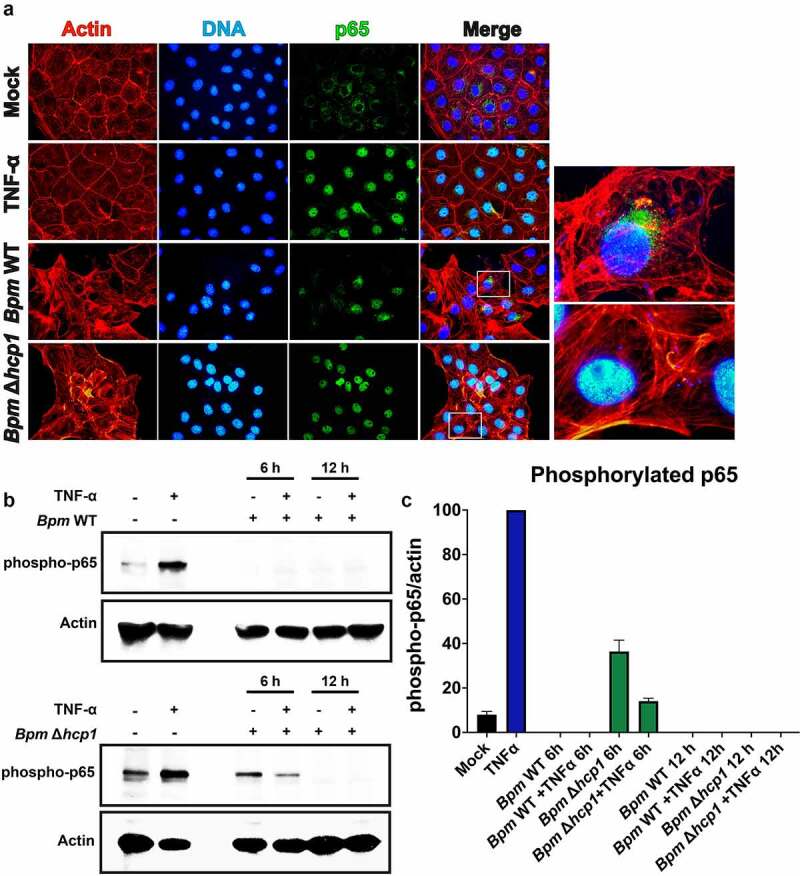
Bpm blocks TNF-α-induced NF-κB activation which is dependent on a functional T6SS. A. Immunofluorescence of NF-κB nuclear translocation in IECs in the presence or absence of Bpm T6SS (Δhcp1), showing sequestration of cytoplasmic NF-κB. The IECs were infected with either Bpm WT or Bpm Δhcp1. After 12 h of infection, cells were fixed, stained, and imaged. Actin cytoskeleton and nuclei were stained with rhodamine phalloidin and DAPI, respectively. For immunofluorescence imaging, treated cells were fixed, permeabilized, and blocked with 1% BSA. NF-κB subunit p65 was detected using an rabbit anti-mouse p65 antibody, followed by goat anti-rabbit IgG antibody conjugated to Alexa-488 fluorophore. Images to the right of bottom two rows represent a magnified view of merged images. Magnified areas are denoted by squares. B. To confirm blockage of NF-κB translocation during Bpm infection and dependency of T6SS, the phosphorylation of NF-κB by TNF-α activation was measured by Western blot. Cell lysates from infected IECs were collected after 6 and 12 h of infection with Bpm WT or Δhcp1. In addition, separate infected cells were treated for 10 min with TNF-α (20 ng/mL) and subjected to lysis. An anti-phosphorylated p65 antibody was used followed by an HRP-conjugated goat anti-mouse IgG. As a positive control, cells were stimulated with TNF-α only. C. Phosphorylation of p65 was quantified for each infection and presented as a ratio phosphorylation p65/actin.
