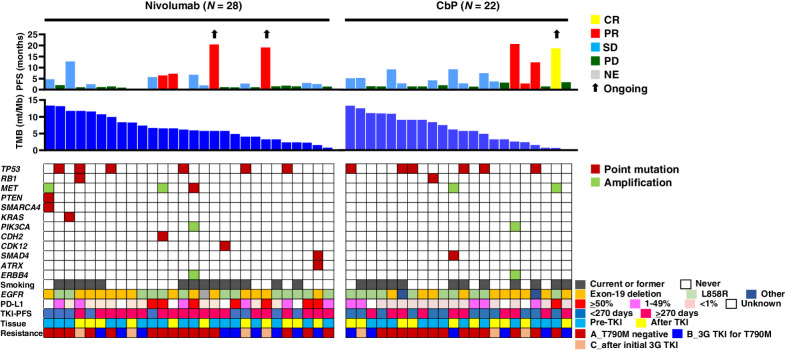
Figure 3.
Individual treatment outcome and response for 50 patients with specimens available for analysis of tumor mutation burden (TMB) according to tumor characteristics. Progression-free survival (PFS) and best objective response (CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, not evaluable) for nivolumab or carboplatin-pemetrexed (CbP) are shown in the top panel. Black arrows indicate ongoing response at data cutoff date. TMB (mutations/Mb) is shown in the middle panel. The distribution of selected gene alterations, smoking status, epidermal growth factor receptor gene (EGFR) mutation type, programmed cell death–ligand 1 (PD-L1) tumor proportion score, PFS for initial EGFR tyrosine kinase inhibitor (TKI) treatment, timing for collection of tumor tissue for analysis (before or after initial EGFR-TKI treatment), and pattern of EGFR-TKI resistance (see legend of Fig. 2 for explanation of A, B, and C) are shown in the bottom panel. 3G, third-generation.