Figure 1.
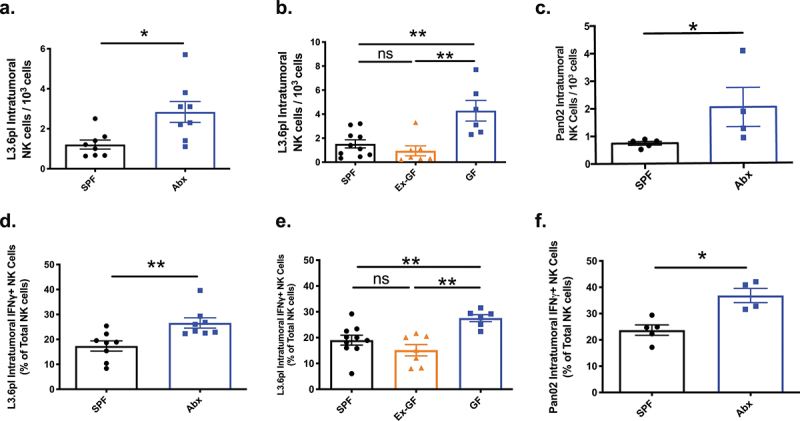
Gut microbiota mediates intratumoral NK cell infiltration and activity in immunocompromised and immunocompetent mice bearing pancreatic cancer xenografts. Heterotopic or orthotopic PDAC xenografts were established as described. At the time of xenograft harvest, tumors were dissociated into single cell suspensions and total NK cell infiltration relative to 104 total tumor cells dissociated were analyzed by flow cytometry. The Rag1−/− mice bearing human L3.6pl PDAC xenografts had decreased NK cell infiltration in specific pathogen-free (SPF) mice compared to those with microbiota depletion with antibiotics (Abx, A). Likewise, SPF-Rag1−/− mice had decreased intratumoral NK cell infiltration compared to germ-free (GF) Rag1−/− mice and the increased intratumoral NK cell infiltration seen in the gnotobiotic mice was reversed with reconstitution of the microbiota of GF-Rag1−/− mice with stool derived from SPF-Rag1−/− mice (“Ex-GF”, B). Finally, the presence of a microbiota in immunocompetent C57BL/6 J mice bearing orthotopic syngeneic Pan02 PDAC xenografts also resulted in decreased intratumoral NK cell infiltration compared to Abx-mediated microbiota depletion in C57BL/6 J mice (c). To determine if differences in NK cell infiltration into PDAC tumors also correlated with differences in activation as measured by interferon gamma (IFNγ) expression, flow cytometry for IFNγ was performed on the intratumoral NK cell population. This demonstrated that the presence of a microbiota also inhibited the activation of intratumoral NK cells in the SPF-Rag1−/− (d, e), Ex-GF-Rag1−/− (e), and SPF-C57BL/6 J models (f) compared to their Abx treated (d, f) or GF (E) cohorts. (*) p < .05, (**) p < .01.
