Figure 3.
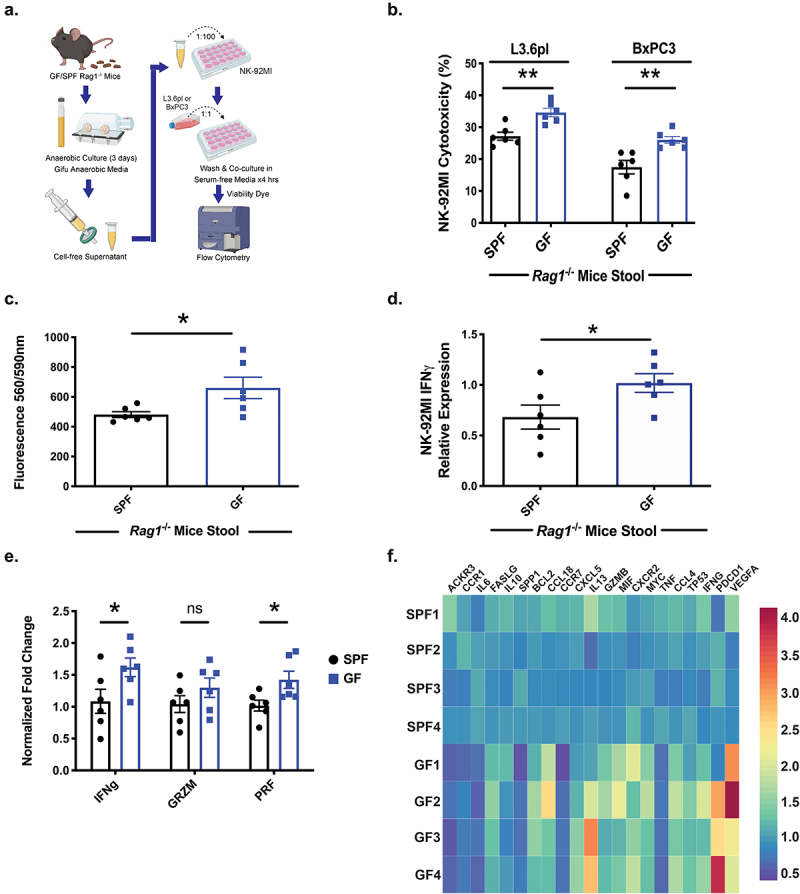
Abiotic bacterial culture supernatant modulates NK cell anti-PDAC activity in vitro. Stool was collected from germ-free (GF) and SPF-Rag1−/− mice and cultured anaerobically for 3 days. Abiotic culture supernatant was prepared after filtration and NK-92MI cells treated with 1% of this supernatant for 3 days. After exposure, NK-92MI cells were harvested and then co-cultured with the human PDAC cell line L3.6pl or BxPC3 for 4 hours and flow cytometry performed to determine cytotoxicity as described (a). NK cells exposed to abiotic culture supernatant from SPF-derived stool had decreased cytotoxicity to L3.6pl and BxPC3 compared to GF-derived abiotic supernatant in vitro (b). Furthermore, NK-92MI cells had a decreased migratory ability in a Boyden chamber assay when pre-treated with abiotic culture supernatant from SPF-Rag1−/− stool (c). These findings of decreased cytotoxicity and decreased migration correlated with decreased activation of NK-92MI cells as measured by flow cytometry for IFNγ(+) NK-92MI cells after exposure to abiotic culture supernatant from SPF-Rag1−/− stool (d). To interrogate pathways responsible for altered cytoxicity, qPCR of treated NK-92MI cells again demonstrated decreased NK cell activation (IFNγ+) but also decreased Perforin expression when exposed to abiotic culture supernatant from SPF stool (e). Furthermore, decreased NK cell cytotoxicity and migration pathways were found via qPCR array as well as increased expression of inhibitory NK cell pathways in NK-92MI cells treated with abiotic culture supernatant from SPF stool (f). (*) p < .05, (**) p < .01.
