Figure 2.
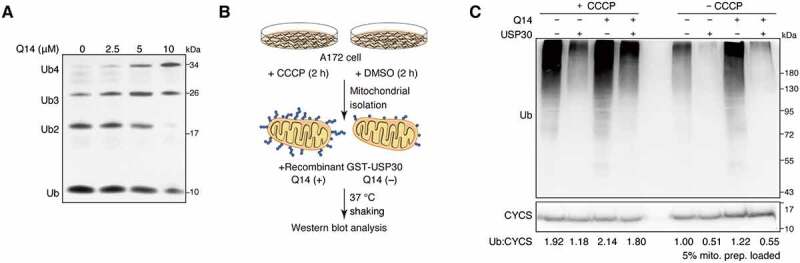
Q14 inhibits the deubiquitinating activities of USP30. (A) Tetrameric ub cleavage inhibition assay. Increasing concentrations of Q14 peptide were incubated with USP30 for 30 min before adding the tetrameric Lys6 ubiquitin chains. After incubating the hydrolysis reaction for 15 min at 37°C, the samples were analyzed by Bis-Tris NuPAGE gels and stained with silver staining. (B) Schematic representation of the experimental setup. Ubiquitinated or normal mitochondria were isolated from CCCP-treated (10 µM, 2 h) or DMSO-treated (2 h) A172 cells. The mitochondrial were incubated with purified GST-USP30 firstly and then peptide Q14 or DMSO were added into them. The reaction was incubated at 37°C with shaking to avoid the mitochondrial settlement. An aliquot for each reaction was used for Western blot analysis to confirm deubiquitination. (C) USP30 and peptide Q14 were incubated with isolated mitochondria that were pre-treated with CCCP or DMSO as shown in Figure 2B. The deubiquitinating activity was assessed by the efficiency of removing the mitochondrial-associated ub chains by Western blot using an anti-ub antibody. A CYCS/cytochrome c antibody was used as a loading control. Representative figure was shown from one of three independent experiments. Full-length gels for A and C are in Fig. S8.
