Figure 7.
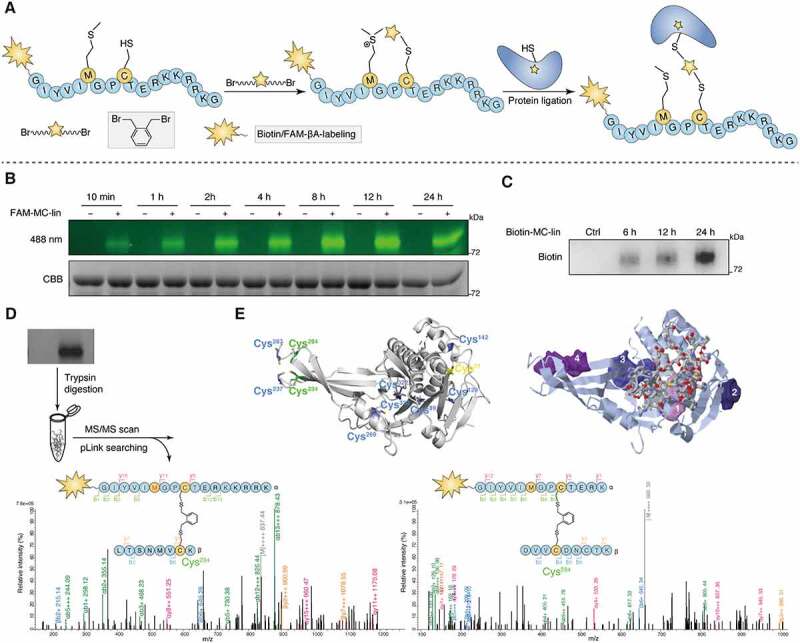
Exploration of the binding site between Q14 and USP30 through proximity promoted protein labeling using Cys-Met bis-alkylated sulfonium tethered peptide. (A) Schematic presentation of the proximity promoted protein labeling using Cys-Met bis-alkylated sulfonium tethered peptide. Mutating the tryptophan and isoleucine to methionine and cysteine separately and Cys-Met bis alkylation was completed by adding the linker o-Xylylene dibromide to peptide. Protein labeling reaction was incubated the alkylated peptide with USP30 protein at 16°C for indicated time. (B) FAM-labeled alkylated peptide (FAM-MC-lin) was incubated with USP30 at 16°C. Sample was collected at indicated time point and analyzed by SDS-PAGE and in-gel fluorescence (488 nm) scanning or Coomassie Brilliant Blue (CBB) staining. (C) Biotin-labeled alkylated peptide (Biotin-MC-lin) was incubated with USP30 at 16°C. Sample was collected at indicated time point and performed by Western blot analysis using anti-biotin antibody. (D) Mass/mass spectrometry analysis using pLink2 for trypsin digested USP30-peptide conjugates indicated that peptide MC-lin binds covalently to C234 and C284 in USP30. Green and red correspond to b- and y- ions of peptide α, respectively. Blue and Orange correspond to b- and y- ions of peptide β. The precursor charge and m/z (gray) are shown in the spectrum. Relative intensity of m/z is plotted. Separate FDR ≤ 5% at PSM level. Variable modification of Oxidation [Met] is in red color. (E) The cysteine distribution of USP30 and potential allosteric binding sites predicted by the online web server CavityPlus. The left figure: all of cysteine in USP30 was colored. The protein labeling cysteines (C234 and C284) were colored green, the enzyme catalytic site cysteine (C77) was colored yellow and other cysteines (C89, C129, C142, C237, C269, C287, C320 and C322) were colored blue. The right figure: prediction of the allosteric binding sites in USP30. The stick region on USP30 indicate the orthosteric site and the other shaded four regions indicate the potential allosteric sites predicted by the online web server CavityPlus. Full-length gels for B and C are in Fig. S8.
