Figure 8.
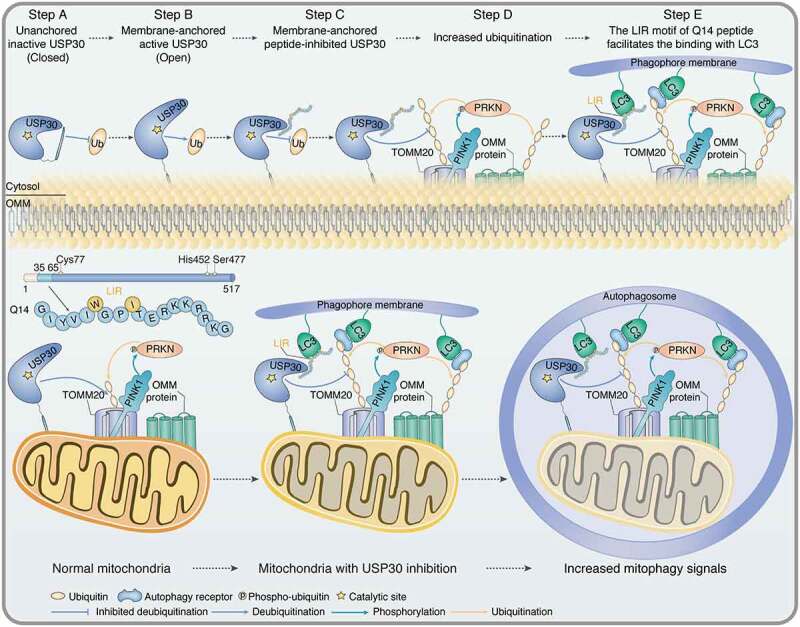
Schematic of the autoinhibitory, mitophagy-inducing model for Q14 peptide. The peptide (Q14) derived from the TM domain of USP30 inhibits its catalytic activity and induces mitophagy. Top: When USP30 is in the cytosol, the TM domain might shield its catalytic center to prevent spurious enzymatic activity (Step A). When the TM domain of USP30 integrates into the OMM, a conformational change triggers exposure of the active catalytic site, thus relieving autoinhibition (Step B). The interaction between Q14 peptide and USP30 deforms the space normally occupied by ubiquitin and prevents the C terminus of ubiquitin from accessing the active site, thus inhibiting USP30 deubiquitinating activity (Step C). Then, Q14 peptide increases ubiquitination of mitochondrial-related proteins by inhibiting the deubiquitinating activity of USP30 (Step D). The LIR motif in Q14 peptide allows it to bind LC3 and accelerate autophagosome formation (Step E). Bottom: Q14 peptide could interact with LC3 and increase mitophagy via the LIR motif. Q14 peptide tethers mitochondrial-anchored USP30 to the autophagosomes by direct binding to USP30 and the key autophagosome protein LC3, further accelerating mitophagy.
