Figure 1.
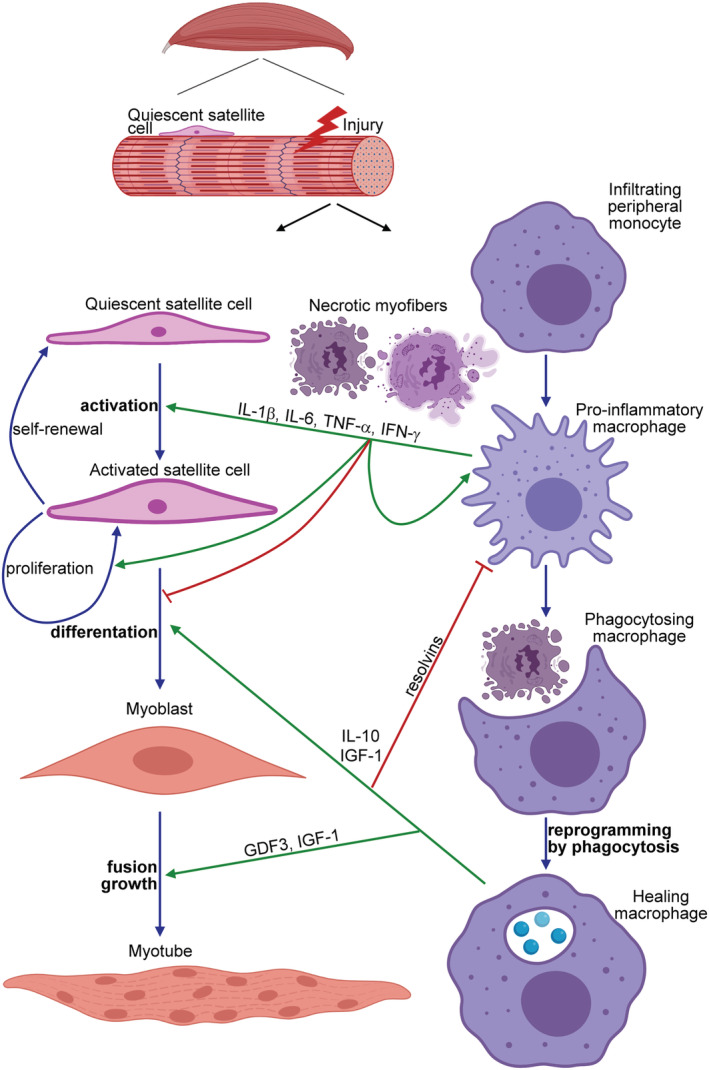
The schematic view of the contribution of macrophages to the myoblast differentiation during skeletal muscle regeneration. Upon induction of necrotic cell death in the skeletal muscle, peripheral monocytes infiltrate the injury site and differentiate into pro‐inflammatory macrophages, and by secreting various pro‐inflammatory cytokines [such as interleukin (IL)‐1β, IL‐6, tumour necrosis factor (TNF)α, or interferon (IFN) γ], they drive the proliferation and differentiation of quiescent satellite cells into myoblasts. In addition, they clear the dead cell debris. Interaction with the apoptotic neutrophils that come first to the injury side but die within hours, and uptake of the dead cells' material during efferocytosis reprogram these pro‐inflammatory macrophages to polarize transcriptionally into healing macrophages. Healing macrophages produce anti‐inflammatory molecules (cytokines and resolvins) to facilitate the resolution of inflammation and growth factors (such as growth differentiation factor 3 and insulin‐like growth factor) to drive the formation of myotubes by promoting both the fusion of myoblasts and the growth of myofibres.
