Figure 2.
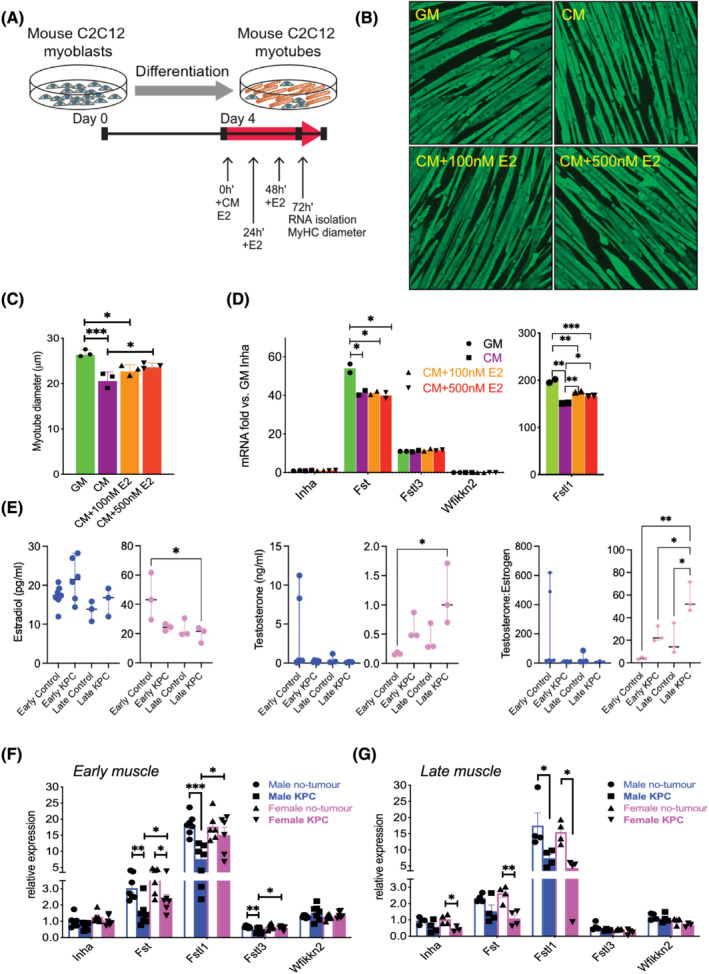
Regulation of Activin pathway members and wasting by PDAC‐produced factors and estradiol (E2). (A) Growth medium (GM) or KPC32908 pancreatic cancer cell‐derived conditioned medium (CM) was added at 30% concentration in differentiation medium (DM), E2 at 100 or 500 nM final concentration to 4‐day‐old differentiated C2C12 myotubes cultured in 12‐well plates, at time 0 h. E2 was replenished at 24 and 48 h. RNA was isolated and myotube diameter was measured at 72 h. (B) C2C12 myotubes were fixed in 1:1 acetone/methanol and blocked in 6% foetal bovine serum phosphate‐buffered saline, followed by incubation with the myosin heavy chain (MyHC) primary antibody and staining with the Alexa Fluor 488‐conjugated secondary antibody. Shown are images acquired at ×4 using the Lionheart LX imager. (C) KPC CM induced atrophy, which was partly blocked by E2. Average myotube diameters were measured from a total of 425–496 myotubes for each group. *P < 0.05, ***P < 0.001. (D) Endogenous Activin inhibitor mRNA expression quantified in C2C12 cultures by real‐time PCR showed Fstl1 was the most abundant among the inhibitors, and was repressed by KPC CM and upregulated by E2. (E) Estradiol (left) and testosterone (middle) concentrations in the plasma of mice were measured by the ELISA method. Testosterone over estradiol ratio was calculated (right). (F) Endogenous Activin inhibitor mRNA in KPC GEMM muscle by real‐time PCR showed sex‐specific and stage‐specific expression in muscle from male and female mice with early‐stage and (G) late‐stage PDAC.
