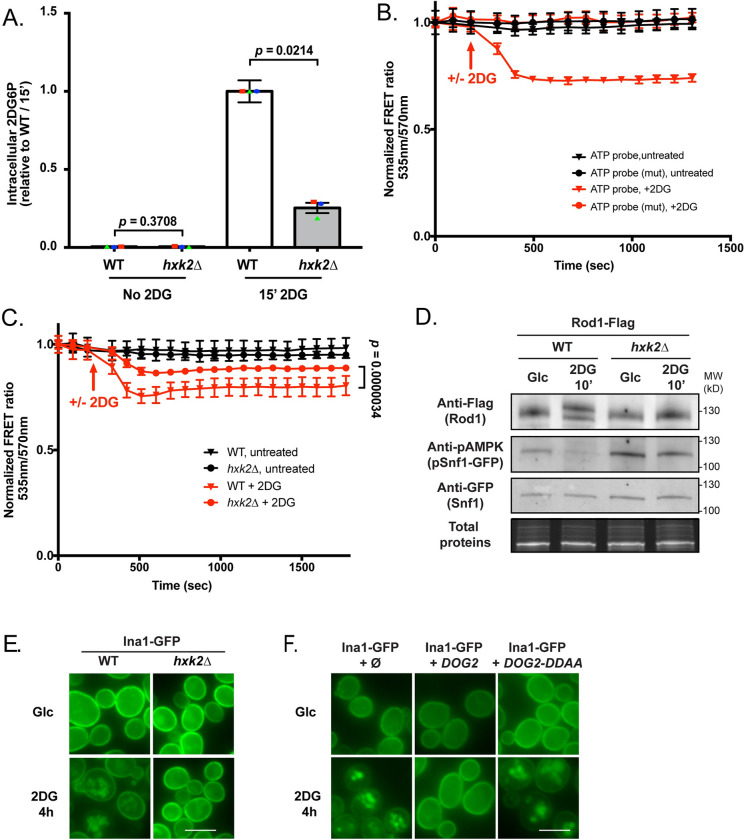
Fig 4. Hexokinase 2 (Hxk2) is the main 2DG-phosphorylating enzyme in vivo.
(A) Intracellular 2DG6P was assayed enzymatically (see Methods) in WT and hxk2Δ cells grown overnight in a glucose-containing medium and treated or not for 15 min with 0.2% 2DG. Values are normalized to the value of the WT / 15 min (n = 3 independent experiments ± SEM, pvalue indicated, paired t-test) (B) 2DG causes a decrease in ATP content as visualized using a FRET ATP biosensor. WT cells expressing the ATP biosensor (WT or mutated version) were grown overnight in a glucose-containing medium and treated with 0.2% 2DG (arrow). The FRET ratio (535/570 nm) was measured over time in a plate reader (see Methods) and is represented as normalized to the t0 value. (n = 3 independent experiments ± SEM). (C) ATP levels were measured as in (B) in WT and hxk2Δ cells in response to 2DG. The FRET ratio (535/570 nm) is represented as normalized to the t0 value (n = 4 independent experiments ± SEM). A paired t-test was used to compare WT + 2DG vs hxk2Δ + 2DG (p-value indicated). (D) Total protein extracts of WT and hxk2Δ expressing Snf1-GFP and Rod1-Flag were prepared before and after 10’ 2DG treatment and immunoblotted using anti-Flag, anti-phospho-AMPK and anti-GFP antibodies. (E) WT or hxk2Δ cells expressing Ina1-GFP were grown in a glucose-containing medium and observed by fluorescence microscopy before and after 2DG treatment for 4h. Scale bar, 5 μm. (F) WT cells expressing Ina1-GFP and transformed with an empty plasmid, or with plasmids allowing the overexpression of DOG2 or its catalytic mutant DOG2-DDAA were grown in a glucose-containing medium and observed by fluorescence microscopy before and after 2DG treatment for 4h. Scale bar, 5 μm.