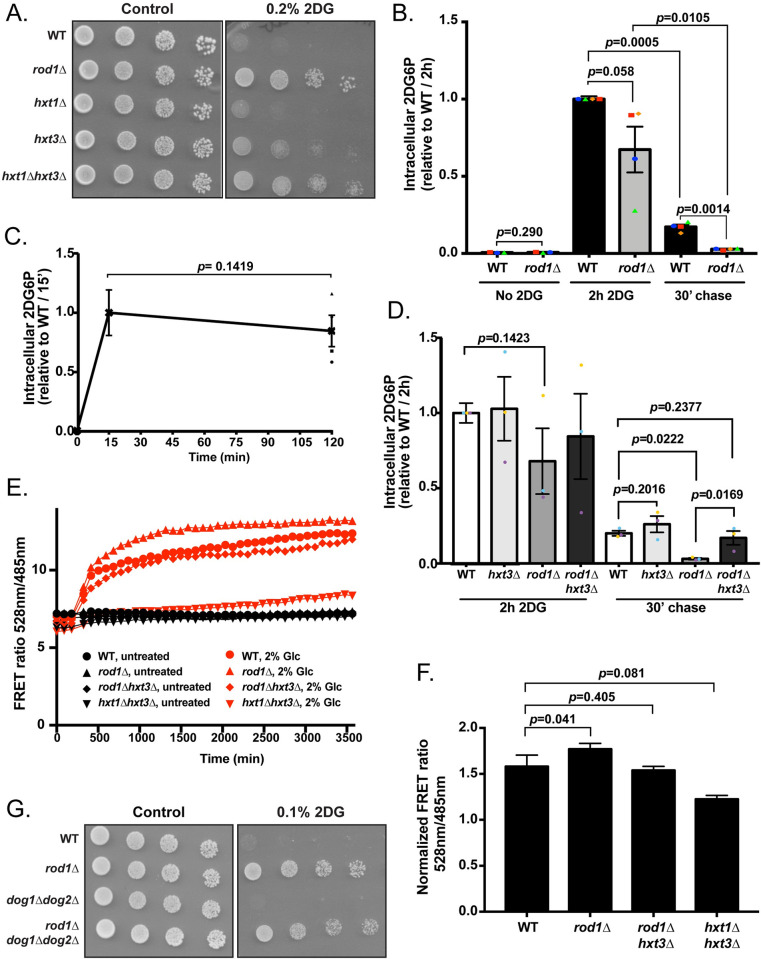
Fig 6. The maintenance of glucose transporters in the rod1Δ mutant promotes 2DG resistance by increasing glucose uptake and limiting 2DG toxicity.
(A) Serial dilutions of cultures of the indicated strains were spotted on SC medium or SC + 0.2% 2DG medium and grown for 4 days at 30°C. (B) Intracellular 2DG6P was assayed enzymatically in WT and rod1Δ cells grown overnight in a glucose-containing medium, treated for 2h with 0.2% 2DG, and then transferred into a 2DG-free glucose-containing medium (“chase”). Values are normalized to the value of the WT / 2h (n = 4 independent experiments ± SEM, paired t-test, p-value indicated). (C) 2DG6P content was assayed from WT cells as in (B) after 15 min and 2h treatment with 0.2% 2DG n = 4 independent experiments ± SEM, paired t-test, p-value indicated). (D) Intracellular 2DG6P was assayed enzymatically in WT, hxt3Δ, rod1Δ and rod1Δ hxt3Δ cells as in (B). Values are normalized to the value of the WT / 2h (n = 3 independent experiments ± SEM, paired t-test, p-value indicated). (E) Intracellular glucose measurement using a FRET-based glucose biosensor (representative experiment). Cells were grown overnight in glucose medium (exponential phase), treated for 4h with 0.2% 2DG, and transferred in a glucose-free buffer. Fluorescence at 485 and 528 nm was measured every 90 sec in a plate reader before and after glucose addition (2%) (see Methods) and the FRET ratio (485/528 nm) is indicated over time. (F) Intracellular glucose was evaluated as in (D) at 2000 sec across n = 5 independent experiments (± SEM, paired t-test) and is represented as normalized to the “before glucose” value. (G) Serial dilutions of cultures of the indicated strains were spotted on SC medium or SC + 0,1% 2DG medium and grown for 4 days at 30°C.