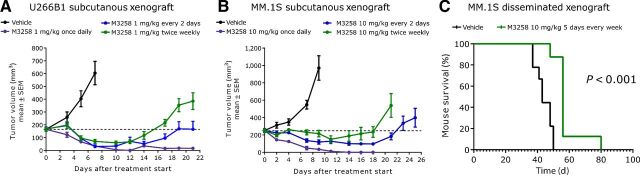
Figure 4.
In vivo efficacy of M3258 in subcutaneous and disseminated multiple myeloma xenograft models. A, Antitumor efficacy of M3258 at 1 mg/kg orally applied either once daily, every 2 days or twice weekly (days 1 and 4) in the U266B1 subcutaneous xenograft model. B, Antitumor efficacy of M3258 at 10 mg/kg orally applied either once daily, every 2 days or twice weekly (days 1 and 4) in the MM.1S subcutaneous xenograft model. For A and B, the percentage tumor/control (% T/C) values for each treatment and the statistical comparison of the efficacy of each group are shown in Supplementary Table S5. C, Mice bearing disseminated MM.1S cells, established via tail vein injection, were treated with M3258 (10 mg/kg, orally, 5 consecutive days of treatment every week) or vehicle and the percentage mouse survival was assessed. The P value for the statistical comparison of survival curves of the vehicle and M3258 groups is indicated.