Abstract
The expression of genes encoding proteins secreted by the SPI1 (Salmonella pathogenicity island) type III secretion apparatus is known to require the transcriptional activators SirA and HilA. However, neither SirA nor HilA is believed to directly activate the promoters of these genes. invF, the first gene of the inv-spa gene cluster, is predicted to encode an AraC-type transcriptional activator and is required for invasion into cultured epithelial cells. However, the genes which are regulated by InvF have not been identified. In this work, an in-frame deletion in invF was constructed and tested for the expression of Φ(sigD-lacZYA), sipC::Tn5lacZY, and a plasmid-encoded Φ(sicA-lacZYA). SigD (Salmonella invasion gene) is a secreted protein required for the efficient invasion of Salmonella typhimurium into cultured eucaryotic cells. sicA (Salmonella invasion chaperone) is the first gene of a putative operon encoding the Sip/Ssp (Salmonella invasion/Salmonella secreted proteins) invasion proteins secreted by the SPI1 type III export apparatus. invF was required for the expression of the sigD, sicA, and sipC fusions. This is the first demonstration that there is a functional promoter in the intergenic sequence between spaS and sicA. In addition, several proteins were either absent from or found in reduced amounts in the culture supernatants of the invF mutant. Therefore, invF is required for the optimal expression of several genes encoding SPI1-secreted proteins. Genetic evidence is also presented suggesting there is HilA-dependent readthrough transcription from the invF promoter at least through sipC.
The expression of genes required for the invasion of eucaryotic cells is stimulated by environmental cues including osmolarity (12), pH (4), and growth state and oxygen tension (10, 27, 38). Several regulators of SPI1 (Salmonella pathogenicity island 1) gene expression have been identified, but it is not known how they recognize environmental signals which affect gene expression. A pho-24 mutant produces constitutively active PhoP, a response regulator which directly or indirectly represses the expression of SPI1 genes (4, 6, 15, 21). One of these genes, hilA, encodes an activator of SPI1 gene expression (3, 28). HilA is believed to directly activate expression from the invF and prgH promoters (3), although this has not been established biochemically. invF is predicted to encode an AraC-type transcriptional activator (for a review, see reference 13), but it is not known which genes it activates (23). invF is not autoregulated, nor does it activate the expression of invH, a gene which encodes an outer membrane lipoprotein component of the SPI1 type III secretion apparatus and which is divergently transcribed from invF (2, 8, 23). Nonetheless, invF is required for efficient invasion of cultured epithelial cells (23), suggesting that it is important for the expression of other genes required for invasion. This hypothesis was tested by examining the effect of an invF mutation on the expression of genes known to be required for invasion.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacterial strains and plasmids used in this study are described in Table 1. Electroporation of plasmids into bacteria was carried out as previously described (36). Plasmids that were manipulated in Escherichia coli were passaged through a restriction-minus (hsd) Salmonella typhimurium LT2 strain (LB5000) (37) prior to electroporation into S. typhimurium SL1344 (19). P22 HT int lysates were harvested and used for transductions as previously described (30).
TABLE 1.
Bacterial strains and plasmids used in this work
| Strain | Genotype | Reference or source |
|---|---|---|
| S. typhimurium | ||
| SL1344 | Wild type | 19 |
| SVM473 | Cmr Φ(sigD-lacZYA)/sigDE+ | This work |
| SB154 | Cmr StrRinvA::Ωcat | J. Galán |
| SVM514 | Strr SprspaS::ΩStr/Sp | This work |
| SVM579 | ΔinvF (in-frame deletion of 465 bp) | This work |
| VV302 | ΔhilA-523 | 3 |
| BJ68 | Tcr; sipC::Tn5lacZY | 34 |
| SVM725 | TcrsipC::Tn5lacZY ΔinvF | This work |
| SVM733 | Strr SprspaS::ΩStr/Sp ΔinvF | This work |
| SVM754 | Strr Spr Tcr ΔinvF spaS::ΩStr/Sp sipC::Tn5lacZY | This work |
| LB5000 | LT2, flaA66 metA22 trp-2 rpsL xyl-401 ilv-452 leu hsd mod+ | 37 |
| Plasmids | ||
| pHG329 | Apr medium-copy-number cloning vector | 39 |
| pWKS130 | Knr, low-copy-number cloning vector | 40 |
| pVV214 | Apr, hilA in pACYC177 | 3 |
| philA | Knr, 2.2-kb NsiI fragment from pVV214 cloned into the PstI site of pWSK130 | This work |
| pHH21 | Apr, sigD-lacZYA, 0.9-kb EcoRI/PstI sigDE promoter and partial coding sequences in pRS415 | 20 |
| pVLT33 | Knr, low-copy-vector for IPTG-inducible expression downstream of Ptac | 9 |
| pHH37 | Knr, 3.2-kb EcoRI-BamHI sigDE fragment in pVLT33 | This work |
| pRW50 | Tcr, low-copy-number transcriptional reporter fusion vector | 29 |
| pHD3 | Tcr, Φ(invF-lacZYA) in pRW50 | This work |
| pHD11 | Tcr, Φ(sicA-lacZYA) in pRW50 | This work |
| pFUSE | Cmr, suicide vector for integration of lacZYA transcriptional reporters onto the chromosome | 5 |
| pHD5 | Cmr, 1-kb sequence including sigDE promoter in pFUSE | This work |
| pDL7-2 | Tcr, cosmid clone containing inv-spa and sicAB genes | 32 |
| pHD7 | Knr, ∼8-kb EcoRI fragment from pDL7-2 containing invHFGEABC′ in pWKS130 | This work |
| pHD8 | Knr, ∼6.7-kb EcoRI fragment from pDL7-2 containing invC′IJ spaOPQRS sicA sequence in pWSK130 | This work |
| pHD9-1 | Knr, 1.7-kb PstI fragment from pHD7 containing invF in pWKS130 | This work |
| pHD10-1 | Apr, 1.7-kb PstI fragment from pHD7 containing invF in pHG329 | This work |
| pHD17 | Cmr, 1.7-kb HindIII invF fragment from pHD10-1 cloned into pACYC184 | This work |
| pHD13 | Apr, same as pHD10-1 but with 465-bp deletion in invF | This work |
| pMAK705 | Cmr, temperature-sensitive origin of replication vector used for gene replacement | 16 |
| pHD15 | Cmr, ∼1.25-kb PstI fragment from pHD13 cloned into pMAK705 | This work |
pDL7-2 (generously provided by Catherine Lee) is a pLAFR2-based clone (11) containing the inv-spa and sicAsipB genes (32). Two subclones were made by digesting pDL7-2 with EcoRI and cloning an ∼8-kb fragment containing invHFGEABC′ and a 6.7-kb fragment containing the invIJ spaOPQRS sicA sequence into pWKS130 (40), resulting in pHD7 and pHD8, respectively. pHD7 was digested with PstI to subclone a 1.7-kb fragment containing invF, creating pHD9-1. This fragment includes about 250 bp of sequence upstream of the invF start codon and is transcribed in the same direction as the lacZ promoter in pWKS130. The same PstI fragment was cloned into the medium-copy-number vector pHG329 (39), forming pHD10-1.
To construct pHD14, a 2.5-kb SalI-BamHI fragment containing spaQRS was cloned from pHD8 into pMAK705 (16). A unique BglII site was used to clone a ∼2-kb streptomycin-spectinomycin resistance cassette from pSmUC into spaS, resulting in pHD14.
To construct the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible sigDE clone pHH37, a 3.2-kb EcoRI-BamHI fragment from pHH20 (20) was cloned into pVLT33 (9). Induction of sigDE expression was done by the addition of IPTG to a final concentration of 100 μM in overnight cultures.
pHD3 (invF-lacZYA) and pHD11 (sicA-lacZYA) were made by PCR amplification (Pfu polymerase; Stratagene) of putative promoter sequences and cloning into pRW50 (29). For pHD3, an invH primer with an EcoRI linker (invH-1 [5′-GGAATTCCGGCGCCATGTTTTTACACAACCGTCAGAAC-3′]) and an invF primer with a BamHI linker (invF-1 [5′-CGGGATCCCGGCAGCTTTTGCGCGGAACACGTCTGTATAAACC-3′]) (Gibco BRL) were used to amplify ∼460 bp between invF and invH from pDL7-2. The resulting fragment was cloned into the BamHI and EcoRI sites of pRW50. For pHD11, the primers spaS-EcoRI-3 (5′-GGAATTCCGCGGAGAAGGTTGGCGTACCTG-3′) and sicA-BamHI-1 (5′-CGGGATCCCGGCGTGGCGCCTTCACTAACGGCATCC-3′) were used to amplify the intergenic sequence (137 bp) between spaS and sicA along with 192 bp of the 3′ end of spaS and 76 bp of sicA. This amplified product was cloned into pRW50 as described above, and both strands were sequenced with the same primers used for amplification to confirm the sequence.
To construct the sigD chromosomal lacZYA reporter, plasmid pFUSE was used (5). pFUSE contains an R6K origin of replication and cannot replicate in SL1344. A 1-kb XbaI fragment from pHH15 (20) containing 0.4 kb of sequence upstream of sigD and 0.6 kb of sigD coding sequence was cloned into the unique XbaI site of pFUSE. Ligations were transformed into E. coli S17-1λpir, and clones were screened by restriction digestion with PstI and EcoRI for inserts in the correct orientation relative to lacZYA. One clone was selected and named pHD5. pHD5 was transferred by conjugation into SVM252 (14028s sirA::Tn10dTc) (20, 22), where it integrated into sigD, leaving an intact sigDE+ copy in addition to a sigD-lacZYA operon fusion. The sirA::Tn10dTc strain was used as a recipient in order to provide a selectable marker (tetracycline resistance) for S. typhimurium. Correct integration of pHD5 was confirmed in several exconjugants by transduction linkage analysis using SVM167 (sigE::Tn10dTc) (86% linkage). The Φ(sigD-lacZYA) fusion was then transduced into the appropriate strains.
Growth conditions.
S. typhimurium and E. coli strains were grown in Luria-Bertani (LB) broth (Difco) at 37°C with aeration on a roller drum or without aeration in standing cultures, as indicated. Antibiotics were used at the following final concentrations: chloramphenicol, 25 μg/ml; kanamycin, 100 μg/ml; streptomycin, 100 μg/ml; spectinomycin, 100 μg/ml; and tetracycline, 15 or 30 μg/ml for single-copy or multicopy tetracycline resistance, respectively. For the detection of β-galactosidase activity, solid medium (LB agar) was supplemented with 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal) at 40 μg/ml. IPTG was used at a final concentration of 100 μM.
Tissue culture invasion assays.
HEp-2 cells were maintained and passaged as recommended by the American Type Culture Collection. For invasion assays, 2 × 105 cells/ml were seeded into Falcon 24-well tissue culture plates (Becton Dickinson, Lincoln Park, N.J.) to obtain about 90% confluent monolayers on the following day. For bacterial cultures, single colonies were inoculated into 2 ml of LB broth and grown for 18 h without shaking at 37°C. Aliquots of 5 μl (107 to 108 CFU) were used per well of tissue culture cells. The invasion assay was performed as previously described (20).
Construction of spaS disruption mutants.
To construct the spaS::ΩStr/Sp mutant SMV514, a 2.5-kb SalI-BamHI fragment containing spaQRS was cloned into pMAK705, creating pHD12. A BamHI streptomycin-spectinomycin resistance cassette from pSmUC was cloned into the unique BglII site within the 5′ region of spaS. This plasmid, pHD14, was used to exchange the disrupted spaS allele with the wild-type allele on the chromosome as previously described (16).
To confirm the disruptions on the chromosome, Southern analysis was performed (36). Labeling of a DNA probe and detection of disrupted sequences were done with enhanced chemiluminescence (ECL). Southern blotting detection reagents (Amersham Pharmacia Biotech). To confirm the spaS disruption, chromosomal DNA was digested with BamHI and probed with a spaS PCR-amplified product. As predicted, the spaS probe hybridized to a 7-kb fragment of SL1344 chromosomal DNA and a 9-kb fragment in the spaS-disrupted strains (data not shown). One spaS::ΩStr/Sp mutant was chosen and called SVM514.
Construction of the invF in-frame deletion mutant.
To make an in-frame deletion in invF, pHD10-1 was digested with ClaI and SacII, the 5′ and 3′ single-stranded overhangs were removed with mung bean nuclease (New England Biolabs), and the blunt ends were ligated with T4 DNA ligase (New England Biolabs). Mung bean nuclease can sometimes remove double-stranded DNA in addition to single-stranded DNA; therefore, several clones were sequenced to identify clones with an in-frame deletion in invF. One clone, pHD13, contained an in frame deletion of 465 bp. A 1.25-kb PstI fragment from pHD13 was subcloned into pMAK705, producing pHD15, which was then used to exchange the deletion onto the chromosome as previously described (16).
To screen for the invF deletion on the wild-type chromosome, bacteria with resolved plasmids were pooled in phosphate-buffered saline, subcultured into LB broth, and infected with P22 HT int containing pHH21 (sigD-lacZYA reporter plasmid, medium copy number) (20). If invF were required for the expression of sigD as predicted, the expression of Φ(sigD-lacZYA) would be reduced in an ΔinvF strain. Transductants were grown on MacConkey lactose agar supplemented with ampicillin. Four of several hundred transductants that formed less red or white colonies were purified and subsequently cured of the reporter plasmid by growing bacteria in LB broth for 5 days, subculturing 1:500 on each day. Individual colonies were screened for ampicillin sensitivity on LB agar containing ampicillin. One mutant was chosen and called SVM579. The invF mutation in this strain was complemented by invF in a low-copy-number vector (pHD9-1), suggesting that the phenotype of this mutant (see Results) was due to the deletion in invF and not another mutation elsewhere on the chromosome. The same technique was used to introduce the invF deletion onto the chromosome of BJ68 (sipC::Tn5lacZY) (34), creating SVM725. The ΔinvF resulted in reduced lacZY expression from the sipC::Tn5lacZY fusion when tested on LB agar plates supplemented with X-Gal.
To confirm the presence of the deletion on the chromosome and the absence of any gross chromosomal rearrangements, Southern analysis was performed (36). Chromosomal DNA was digested with PstI and probed with the 1.7-kb PstI fragment from pHD10-1. The probe hybridized to a 1.7-kb PstI fragment of chromosomal DNA from SL1344 and a 1.2- to 1.3-kb fragment of SVM579 and SVM725 as predicted (data not shown).
To construct the ΔinvF spaS::ΩStr/Sp sipC::Tn5lacZY triple mutant, SVM754, the spaS::ΩStr/Sp mutation in SVM514 was transduced into SVM579 (ΔinvF). Nine purified transductants were screened by Southern hybridization to confirm that the invF deletion had not been lost upon transduction due to its spaS-linked location on the chromosome (data not shown). One mutant, SVM733 (ΔinvF spaS::ΩStr/Sp), was used to make a P22 HT int lysate. The spaS::ΩStr/Sp mutation from SVM733 was transduced into SVM725 (ΔinvF sipC::Tn5lacZY). Tetracycline-, spectinomycin-, and streptomycin-resistant transductants were purified and checked for P22 sensitivity.
Analysis of culture supernatants.
Cultures were grown in 5 to 10 ml of LB broth with antibiotics for 18 h without aeration, and equivalent units of optical density at 600 nm were harvested as previously described (20). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (26) on 7.5% gels for silver stain analysis (7) and 5% gels for immunoblot (Western) analysis (ECL Western blotting detection system; Amersham Pharmacia Biotech). It is notable that in these gels (30% acrylamide to 1.6% bisacrylamide), some proteins migrated more slowly than through gels poured with 0.8% acrylamide (data not shown). For immunoblots, proteins were transferred onto Immobilon-P polyvinylidene difluoride membranes (Millipore).
Antibodies.
To make antibodies to SigD, the first 575 bp of sigD were cloned into the expression vector pMAL-c1 (New England Biolabs). Expression and affinity purification of the maltose binding protein (MBP)-SigD′ fusion protein expressed from this plasmid were performed as described by the manufacturer. Protein eluted from the amylose column was separated on a 7.5% SDS-polyacrylamide gel, and the fusion protein was excised from the gel. Lyophilized gel slices containing MBP-SigD′ were used to raise rabbit antibodies to SigD (Covance Research Services, Denver, Pa.). The anti-SigD antibodies were preadsorbed with an acetone powder of E. coli DH5α expressing MBP (17) to reduce cross-reactivity of the antibody to proteins other than SigD. The preadsorbed antiserum was used at a 1:1,000 dilution. Anti-rabbit horseradish peroxidase-conjugated secondary antibodies were obtained from Sigma (St. Louis, Mo.).
Enzyme assays.
β-Galactosidase assays were performed and values were calculated as previously described (31). Cultures were grown in 5 ml of LB with appropriate antibiotics for 18 h at 37°C in 13- by 100-mm screw-capped tubes. Samples of 1.5 ml were harvested, washed once in 0.88% NaCl, and resuspended in 1 ml of working buffer; 100 μl of each cell suspension was used per assay.
Sequence analysis.
Sequencing was performed by using the BigDye Terminator Cycle Sequencing Ready Reaction system (PE Applied Biosystems, Foster City, Calif.). Reactions were analyzed by the Washington University Nucleic Acid Chemistry Laboratory (St. Louis, Mo.). Sequence analyses (homologies, mapping, etc.) were performed using the Wisconsin Sequence Analysis Package (Genetics Computer Group, Inc.).
RESULTS
InvF is essential for the expression of effector genes.
invF is the first open reading frame of a large gene cluster encoding components of the SPI1 type III secretion apparatus (23). It has been proposed that InvF activates the expression of genes required for invasion (23), but this has not been tested. Therefore, a strain with an in-frame deletion of 465 bp in invF, SVM579, was constructed and tested for expression of the Φ(sigD-lacZYA) (chromosomal), sipC::Tn5lacZY, and Φ(sicA-lacZYA) (episomal) reporters. sigD, which is unlinked to SPI1, encodes a protein secreted by the SPI1 type III secretion apparatus and is required for efficient invasion into cultured epithelial cells (20). The SigD homologue in S. dublin, SopB (Salmonella outer protein), has been implicated as a factor important for causing enteritis in a calf model of infection (14). Contrary to our previous report (20), sigD expression was found to be hilA dependent (1). Therefore, strain VV302 (ΔhilA-523) was resent to us and retested for the expression of Φ(sigD-lacZYA) in pHH21. In addition to the episomal sigD-lacZYA fusion, a chromosomal reporter fusion, Φ(sigD-lacZYA)/sigDE+, was constructed and tested in the wild-type and VV302 strains. The expression of both Φ(sigD-lacZYA) fusions was significantly reduced in the hilA background (data not shown). Thus, although the hilA deletion in VV302 in our previous work was confirmed by Southern analysis and by tissue culture invasion assays (20), it appeared that the original strain of VV302 we received had acquired a suppressing mutation which allowed for hilA-independent expression of Φ(sigD-lacZYA).
sicA is predicted to encode a chaperone for one or more of the secreted proteins encoded in SPI1 (sip/ssps) and is believed to be cotranscribed with these genes (18, 21, 24, 25). The expression of Φ(sigD-lacZYA) and Φ(sicA-lacZYA) in SVM579 was found to be much lower than in the wild-type strain SL1344 (Table 2). The regulation defect due to the invF deletion was complemented by pHD9-1 (invF+), demonstrating that the regulation phenotype was due to ΔinvF. Expression of the Φ(sicA-lacZYA) and Φ(sigD-lacZYA) reporters was not complemented by philA (hilA+) (Table 2), suggesting that the regulatory effect of InvF on the sigD and the sicA promoters is downstream of HilA.
TABLE 2.
Complementation of the ΔinvF mutant for regulation of gene expressiona
| Strain | β-Galactosidase activityb (U)
|
||
|---|---|---|---|
| pWKS130 | pHD9 | philA | |
| SVM473 [Φ(sigD-lacZYA)/sigDE+] | 74 | 580 | 1,847 |
| SVM579 [ΔinvF Φ(sigD-lacZYA)/sigDE+] | 3 | 642 | 5 |
| SL1344/pHD11 [Φ(sicA-lacZYA)] | 114 | 986 | 3,612 |
| SVM579 (ΔinvF)/pHD11 [Φ(sicA-lacZYA)] | 2 | 1,095 | 5 |
| BJ68 (sipC::Tn5lacZY) | 962 | 4,473 | 6,277 |
| SVM725 (BJ68ΔinvF) | 196 | 4,268 | 1,291 |
| SVM754 (BJ68ΔinvF spaS::ΩStr/Sp) | 9 | 4,430 | 16 |
See Table 1 for descriptions of strains and plasmids.
Average of duplicate β-galactosidase assays performed on duplicate cultures, representative of several independent assays. The standard deviation of β-galactosidase activity was less than 15% between cultures except for BJ68/pWKS130, in which case it was 24%.
The expression of a sipC::Tn5lacZY chromosomal reporter fusion from strain BJ68 (34) was also tested in the ΔinvF background. The expression of sipC::Tn5lacZY was significantly reduced in the ΔinvF mutant (Table 2). This regulatory defect could be complemented by pHD9-1 (invF+). Interestingly, hilA provided in multicopy could also increase the expression of sipC::Tn5lacZY, suggesting that sipC expression could be activated from either a HilA- or an InvF-dependent promoter (Table 2). The HilA-dependent expression of sipC::Tn5lacZY in the ΔinvF mutant could be virtually eliminated by a polar disruption in spaS, a gene upstream of sicA and sipC. In contrast, the InvF-dependent expression of sipC::Tn5lacZY was not affected by the polar disruption in spaS. These results suggest the production of a readthrough transcript beginning upstream of spaS that is HilA dependent and a second transcript beginning downstream of spaS (probably the sicA promoter) that is InvF dependent (Table 2).
Expression from the invF promoter was measured from pHD3 (invF-lacZYA) in SVM579 to determine if invF was autoregulated. As previously reported for a different invF mutant (23), the in-frame deletion in invF did not significantly affect expression of the invF promoter (64 (wild type) versus 53 [ΔinvF] U of β-galactosidase activity).
InvF is not sufficient to activate the expression of Φ(sicA-lacZYA) or Φ(sigD-lacZYA) in E. coli.
Previous work demonstrated that an Φ(invF-lacZYA) reporter (pVV562) could be activated by hilA (in pVV214) expressed in E. coli (3). To determine if HilA and/or InvF directly activates sigD or sicA expression, the effect of providing hilA or invF in trans on the expression of Φ(sigD-lacZYA) in pHH21 (20) and of Φ(sicA-lacZYA) in pHD11 was tested in E. coli. Although philA and pVV214 (data not shown) were able to activate Φ(invF-lacZYA) (pHD3) in E. coli, neither pVV214 nor philA could activate Φ(sicA-lacZYA) or Φ(sigD-lacZYA), respectively, in E. coli (Table 3). Expression of these fusions was also tested in the presence of invF. Like hilA, invF, with or without hilA, was unable to activate the expression of either fusion in E. coli (Table 3). Therefore, invF may require additional factors or signals that are absent from E. coli for the activation of the sigD and the sicA promoters.
TABLE 3.
Expression of Φ(invF-lacZYA), Φ(sigD-lacZYA), and Φ(sicA-lacZYA) in E. colia
| Reporter genotype (name) | β-Galactosidase activityb (U)
|
||
|---|---|---|---|
| hilA+ | invF+ | hilA+invF+ | |
| Φ(invF-lacZYA) (pHD3)c | 2,798 | ND | ND |
| Φ(sigD-lacZYA) (pHH21)d | 84 | 89 | 81 |
| Φ(sicA-lacZYA) (pHD11)d | <1 | <1 | <1 |
See Table 1 for descriptions of plasmids.
Average of duplicate β-galactosidase assays performed per culture, representative of several assays done on different days. The standard deviation of β-galactosidase activity was less than 10% between assays. ND, not determined.
The control strain, DH5α containing pHD3 and the cloning vector pWSK130, produced 2 U of β-galactosidase activity. hilA was provided by philA.
The amount of β-galactosidase activity produced by strains containing the cloning vector pWSK130 was comparable to the values obtained with hilA provided in trans. Due to plasmid incompatibility constraints, different hilA and invF constructs were used. For the pHH21 assays, hilA was provided by philA and invF was provided by pHD17 (Table 1). For the pHD11 assays, hilA was provided by pVV214 and invF was provided by pHD9-1.
InvF is required for efficient invasion into cultured epithelial cells.
Invasion into cultured epithelial cells was also quantitated for the SVM579 mutant and wild-type S. typhimurium. Invasion of HEp-2 cells by the invF deletion strain was reduced significantly (Table 4), but not to the extent previously reported (23). It is possible that the invF mutation tested by Kaniga et al. (23) was having a polar effect on the expression of the inv genes and thus had a stronger phenotype. Moreover, invasion by the ΔinvF mutant was not as reduced as invasion by a secretion-defective invA (SB154) or spaS (SVM514) mutant (Table 4). Invasion by SVM579 into HEp-2 cells was fully complemented to wild-type levels by pHD9-1. Interestingly, invasion was partially restored by philA (Table 4).
TABLE 4.
Complementation of the ΔinvF mutant for invasion of HEp-2 cells
| Strain | Mean % invasion ± SDa (% of wild-type level)
|
|||
|---|---|---|---|---|
| No plasmid | pWKS130 (vector) | pHD9 (invF+) | philA | |
| SL1344 | 12.4 ± 0.3 (100) | 7.7 ± 1.4 (100) | 7.1 ± 1.3 (100) | 8.0 ± 2.0 (100) |
| SVM579 (ΔinvF) | 0.6 ± 0.1 (5) | 0.6 ± 0.1 (7) | 6.8 ± 1.3 (96) | 3.2 ± 0.3 (40) |
| SB154 (invA::Ωcat) | 0.02 ± 0.006 (0.1) | ND | ND | ND |
| SVM514 (spaS::ΩStr/Sp) | 0.02 ± 0.001 (0.1) | ND | ND | ND |
After bacteria were allowed to invade during a 1-h incubation. Values are representative of several assays done in duplicate. ND, not determined.
Analysis of secreted proteins from the invF mutant.
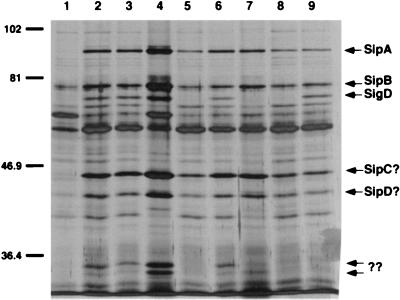
Proteins that are secreted by the SPI1 type III machinery are believed to be the effectors which stimulate the uptake of bacteria by eucaryotic host cells (18, 20, 21, 24, 25). Because the expression of genes encoding several of these proteins was reduced in the invF mutant, the secreted protein profile of SVM579 was analyzed by SDS-PAGE and silver staining. Compared to the wild-type strain, several proteins were missing from the culture supernatants of the invF mutant, including SigD (confirmed by immunoblotting with anti-SigD antibodies [data not shown]) and a smaller protein migrating at about 36 kDa (Fig. 1, lanes 2 and 5) (it is notable that most of the known proteins, including SipA, SipB, and SigD, did not migrate according to their predicted molecular weights in these gels [see Materials and Methods]). Unexpectedly, Sip/SspA was clearly observed in culture supernatants of the invF mutant, although in slightly lower amounts, compared to the wild-type supernatant proteins. Sip/SspA and the other SPI1 secreted proteins were clearly absent from the supernatants of the spaS mutant SVM514 (Fig. 1, lane 1). pHD9-1 restored the secretion of SigD and the ∼36-kDa protein into culture supernatants of the invF mutant and increased Sip/Ssp secretion to wild-type levels (Fig. 1, lane 6).
FIG. 1.
Supernatant proteins from wild-type and ΔinvF strains. Lane 1, supernatant proteins from the secretion defective spaS mutant SVM514; lanes 2 to 7, S. typhimurium SL1344 containing pWSK130, pHD9 (invF+), and philA (lanes 2 to 4) and the invF mutant SVM579 containing the same plasmids in the same order (lanes 5 to 7); lanes 8 and 9, supernatant proteins from SVM579 strains containing the vector pVLT33 (lane 8) and the IPTG-inducible sigDE clone pHH37 (lane 9). Positions of molecular weight standards are indicated in kilodaltons on the left; previously identified secreted proteins are indicated on the right. Question marks denote proteins that have not been confirmed by immunoblot analysis. Proteins were prepared and analyzed as described in Materials and Methods.
Unlike pHD9-1, philA did not restore SigD or the ∼36-kDa protein into the culture supernatants of the ΔinvF mutant. However, philA did increase the amounts of several other proteins including the Sip/Ssps to culture supernatants of the ΔinvF mutant (Fig. 1, lane 7). Because philA could increase the expression of sipC::Tn5lacZY in the ΔinvF mutant, it is not surprising that the Sip/Ssp proteins in culture supernatants are increased accordingly (Fig. 1, lane 7). This result may explain why philA partially restores invasion of an invF mutant into tissue culture cells.
To determine if InvF was required for the secretion of SigD in addition to the regulation of sigDE expression, an inducible clone of sigDE, pHH37 (Ptac-sigDE), was transformed into SVM579. If invF were required for secretion, SigD would not appear in culture supernatants even after the induction of sigDE expression with IPTG. The secretion of SigD was clearly restored to the supernatants of the invF mutant containing pHH37, demonstrating that InvF is not required for the secretion of proteins by the SPI1 type III secretion system (Fig. 1, lane 9).
DISCUSSION
invF was identified by sequence analysis of the SPI1 region and was predicted to encode an AraC-type transcriptional activator (23). However, InvF-dependent genes were not identified. In this study, invF was shown to be required for the expression of Φ(sigD-lacZYA) and Φ(sicA-lacZYA) fusions. An in-frame invF deletion mutant was fully complemented for the expression of these reporters by invF cloned into a low-copy-number vector but was not complemented by hilA, a central activator of SPI1 gene expression (3). In contrast, although a sipC::Tn5lacZY reporter also required invF for optimal expression, hilA provided in multicopy was also able to increase sipC expression in the ΔinvF mutant.
Several of the genes encoding secreted proteins, specifically the sip/ssp genes, are immediately downstream of the inv-spa gene cluster (18, 21, 24, 25). Because the sip/ssp locus is only 137 bp downstream of the spa genes, it was possible that sip/ssp expression could be activated from the invF promoter ∼12 kb upstream of sicA; no transcriptional terminator is evident in this intergenic region. This observation is supported by the analysis of secreted proteins from the invF mutant which revealed the presence, arguably in lesser amounts, of several of the Sip/Ssp proteins. Moreover, these proteins could be restored to wild-type levels in the invF mutant by providing hilA on a low-copy-number plasmid. hilA could also partially complement the invasion defect of the invF mutant. Most importantly, the expression of sipC::Tn5lacZY could be activated in an invF mutant with the addition of hilA in multicopy, and this expression could be eliminated by a polar disruption in spaS. These results suggest that readthrough expression of the sip/ssp genes could be activated from the invF promoter by HilA.
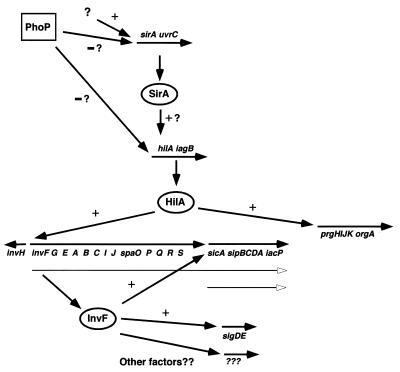
To determine if a promoter was present immediately upstream of the sip/ssp gene cluster, an episomal, Φ(sicA-lacZYA) reporter fusion in a low-copy-number vector was made. Expression of this fusion was dramatically reduced in the invF mutant. The regulation defect was complemented by invF but not hilA. This result demonstrates for the first time the presence of an InvF-dependent promoter immediately upstream of sicA. From these results taken together with the invasion assays, the β-galactosidase assays, and the SDS-PAGE data, it appears that expression of the sip/ssp genes can be driven in part from the HilA-dependent invF promoter in addition to an InvF-dependent promoter immediately upstream of sicA (Fig. 2).
FIG. 2.
Model for the regulation of invasion/virulence gene expression in S. typhimurium. The direction of transcription for each gene cluster is indicated by closed arrows; open arrows represent putative transcripts of the inv-spa and sip/ssp genes. Question marks indicate either unidentified regulatory factors or unclear relationships between the designated regulator and the noted promoter.
The expression of a Φ(sigD-lacZYA) fusion in the invF deletion mutant was also dramatically reduced. SigD was absent from culture supernatants of the ΔinvF mutant and restored by pHD9-1 (invF+). In contrast to the Sip/Ssp proteins, SigD was not restored to culture supernatants when philA was placed in the invF mutant. In addition to SigD, a ∼36-kDa protein was absent from invF mutant culture supernatants. This protein was also restored by the presence of invF but not hilA on a low-copy-number plasmid. Therefore, unlike the sip/ssp genes, sigD (and possibly the ∼36-kDa protein) is not likely to be directly dependent on hilA for expression. It is notable that unlike the case for hilA, providing invF in multicopy does not result in the hypersecretion of proteins found in the culture supernatants of the wild-type strain (Fig. 1, lane 3 versus lane 4). Although InvF activates the expression of genes encoding secreted proteins, it probably does not increase the transcription of the apparatus genes required for the secretion of these proteins. This may explain why the hypersecretion of proteins does not occur despite the hyperexpression of the effector genes observed when invF is present in multicopy.
It is clear that the regulation of SPI1 gene expression is complicated and multifactorial (Fig. 2). hilA expression is dependent, directly or indirectly, on SirA, a protein which is known to be conserved in several of the Enterobacteriaceae (22, 33, 35). HilA, a member of the OmpR/ToxR family of regulators, in turn activates the expression of genes encoding the type III secretion apparatus (3). This effect is predicted to be direct because hilA expressed in E. coli can activate the expression of either Φ(invF-lacZYA) or Φ(prgH-lacZYA) (3). This work provides the first demonstration that the AraC-type transcriptional activator InvF is required for the expression of genes encoding proteins secreted by the type III secretion system. Further analysis will be necessary to determine if InvF itself binds to sequences upstream of sicA and sigD or if it activates the expression of another gene required for their expression. Because invF and hilA cannot activate the expression of Φ(sicA-lacZYA) or Φ(sigD-lacZYA) in E. coli, it seems unlikely that InvF alone is sufficient for activation. Perhaps InvF, like AraC, requires a cofactor (in the case of AraC, arabinose) (13) which induces a conformational change in InvF allowing it to bind to the appropriate promoters. Future studies will elucidate if and how InvF directly interacts with the promoters of genes encoding secreted effectors.
ACKNOWLEDGMENTS
We thank Andrew Darwin for critically reviewing the manuscript. We also thank Brad Jones and Catherine Lee for strains indispensable for this work. We especially thank C. Lee for helpful and open discussions about this project.
This work was supported by National Institutes of Health grant AI01230 to V.L.M.
REFERENCES
- 1.Ahmer B M M, van Reeuwijk J, Watson P R, Wallis T S, Heffron F. Salmonella SirA is a global regulator of genes mediating enteropathogenesis. Mol Microbiol. 1999;31:971–982. doi: 10.1046/j.1365-2958.1999.01244.x. [DOI] [PubMed] [Google Scholar]
- 2.Altmeyer R M, McNern J K, Bossio J C, Rosenshine I, Finlay B B, Galán J E. Cloning and molecular characterization of a gene involved in Salmonella adherence and invasion of cultured epithelial cells. Mol Microbiol. 1993;7:89–98. doi: 10.1111/j.1365-2958.1993.tb01100.x. [DOI] [PubMed] [Google Scholar]
- 3.Bajaj V, Hwang C, Lee C A. hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol Microbiol. 1995;18:715–727. doi: 10.1111/j.1365-2958.1995.mmi_18040715.x. [DOI] [PubMed] [Google Scholar]
- 4.Bajaj V, Lucas R L, Hwang C, Lee C A. Co-ordinate regulation of Salmonella typhimurium invasion genes by environmental and regulatory factors is mediated by control of hilA expression. Mol Microbiol. 1996;22:703–714. doi: 10.1046/j.1365-2958.1996.d01-1718.x. [DOI] [PubMed] [Google Scholar]
- 5.Baumler A J, Tsolis R M, van der Velden A W M, Stojiljkovik I, Anic A, Heffron F. Identification of a new iron regulated locus of Salmonella typhi. Gene. 1996;183:207–213. doi: 10.1016/s0378-1119(96)00560-4. [DOI] [PubMed] [Google Scholar]
- 6.Behlau I, Miller S I. A PhoP-repressed gene promotes Salmonella typhimurium invasion of epithelial cells. J Bacteriol. 1993;175:4475–4484. doi: 10.1128/jb.175.14.4475-4484.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blum H, Beier H, Gross H J. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis. 1987;8:93–99. [Google Scholar]
- 8.Daefler S, Russel M. The Salmonella typhimurium InvH protein is an outer membrane lipoprotein required for the proper localization of InvG. Mol Microbiol. 1998;28:1367–1380. doi: 10.1046/j.1365-2958.1998.00908.x. [DOI] [PubMed] [Google Scholar]
- 9.de Lorenzo V, Eltis L, Kessler B, Timmis K N. Analysis of Pseudomonas gene products using lacIq/Ptrp-lac plasmids and transposons that confer conditional phenotypes. Gene. 1993;123:17–24. doi: 10.1016/0378-1119(93)90533-9. [DOI] [PubMed] [Google Scholar]
- 10.Ernst R K, Dombroski D M, Merrick J M. Anaerobiosis, type 1 fimbriae, and growth phase are factors that affect invasion of HEp-2 cells by Salmonella typhimurium. Infect Immun. 1990;58:2014–2016. doi: 10.1128/iai.58.6.2014-2016.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman A M, Long S R, Brown S E, Buikema W J, Ausubel F M. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982;18:289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- 12.Galán J E, Curtiss R., III Expression of Salmonella typhimurium genes required for invasion is regulated by changes in DNA supercoiling. Infect Immun. 1990;58:1879–1885. doi: 10.1128/iai.58.6.1879-1885.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallegos M-T, Schleif R, Bairoch A, Hofman K, Ramos J L. AraC/XylS family of transcriptional regulators. Microbiol Mol Biol Rev. 1997;61:393–410. doi: 10.1128/mmbr.61.4.393-410.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galyov E E, Wood M W, Rosqvust R, Mullan P B, Watson P R, Hedges S, Wallis T S. A secreted effector protein of Salmonella dublin is translocated into eucaryotic cells and mediates inflammation and fluid secretion in infected ileal mucosa. Mol Microbiol. 1997;25:903–912. doi: 10.1111/j.1365-2958.1997.mmi525.x. [DOI] [PubMed] [Google Scholar]
- 15.Gunn J S, Hohmann E L, Miller S I. Transcriptional regulation of Salmonella virulence: a PhoQ periplasmic mutation results in increased net phosphotransfer in phoP. J Bacteriol. 1996;178:6369–6373. doi: 10.1128/jb.178.21.6369-6373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamilton C M, Aldea M, Washburn B K, Babitzke P, Kushner S R. New method for generating deletions and gene replacements in Escherichia coli. J Bacteriol. 1989;171:4617–4622. doi: 10.1128/jb.171.9.4617-4622.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 18.Hermant D, Ménard R, Arricau N, Parsot C, Popoff M Y. Functional conservation of the Salmonella and Shigella effectors of entry into epithelial cells. Mol Microbiol. 1995;17:781–789. doi: 10.1111/j.1365-2958.1995.mmi_17040781.x. [DOI] [PubMed] [Google Scholar]
- 19.Hoiseth S K, Stocker B A D. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 20.Hong K H, Miller V L. Identification of a novel Salmonella invasion locus homologous to Shigella ipgDE. J Bacteriol. 1998;180:1793–1802. doi: 10.1128/jb.180.7.1793-1802.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hueck C J, Hantman M J, Bajaj V, Johnston C, Lee C A, Miller S I. Salmonella typhimurium secreted invasion determinants are homologous to Shigella Ipa proteins. Mol Microbiol. 1995;18:479–490. doi: 10.1111/j.1365-2958.1995.mmi_18030479.x. [DOI] [PubMed] [Google Scholar]
- 22.Johnston C, Pegues D A, Hueck C J, Lee C A, Miller S I. Transcriptional activation of Salmonella typhimurium invasion genes by a member of the phosphorylated response-regulator superfamily. Mol Microbiol. 1996;22:715–727. doi: 10.1046/j.1365-2958.1996.d01-1719.x. [DOI] [PubMed] [Google Scholar]
- 23.Kaniga K, Bossio J C, Galán J E. The Salmonella typhimurium invasion genes invF and invG encode homologues of the AraC and PulD family of proteins. Mol Microbiol. 1994;13:555–568. doi: 10.1111/j.1365-2958.1994.tb00450.x. [DOI] [PubMed] [Google Scholar]
- 24.Kaniga K, Trollinger D, Galán J E. Identification of two targets of the type III protein secretion system encoded by the inv and spa loci of Salmonella typhimurium that have homology to the Shigella IpaD and IpaA proteins. J Bacteriol. 1995;177:7078–7085. doi: 10.1128/jb.177.24.7078-7085.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaniga K, Tucker S, Trollinger D, Galán J E. Homologs of the Shigella IpaB and IpaC invasins are required for Salmonella typhimurium entry into cultured epithelial cells. J Bacteriol. 1995;177:3965–3971. doi: 10.1128/jb.177.14.3965-3971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Lee C A, Falkow S. The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. Proc Natl Acad Sci USA. 1990;87:4304–4308. doi: 10.1073/pnas.87.11.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee C A, Jones B D, Falkow S. Identification of a Salmonella typhimurium invasion locus by selection for hyperinvasive mutants. Proc Natl Acad Sci USA. 1992;89:1847–1851. doi: 10.1073/pnas.89.5.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lodge J, Fear J, Busby S, Gunasekaran P, Kamini N R. Broad host range plasmids carrying the Escherichia coli lactose and galactose operons. FEMS Microbiol Lett. 1992;95:271–276. doi: 10.1016/0378-1097(92)90441-p. [DOI] [PubMed] [Google Scholar]
- 30.Maloy S R, Stewart V J, Taylor R K. Genetic analysis of pathogenic bacteria. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. [Google Scholar]
- 31.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 32.Mills D M, Bajaj V, Lee C A. A 40 kb chromosomal fragment encoding Salmonella typhimurium invasion genes is absent from the corresponding region of the Escherichia coli K-12 chromosome. Mol Microbiol. 1995;15:749–759. doi: 10.1111/j.1365-2958.1995.tb02382.x. [DOI] [PubMed] [Google Scholar]
- 33.Moolenar G F, van Sluis C A, Backendorf C, van de Putte P. Regulation of the Escherichia coli excision repair gene uvrC. Overlap between the uvrC structural gene and the region coding for a 24 kD protein. Nucleic Acids Res. 1987;15:4273–4289. doi: 10.1093/nar/15.10.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penheiter K L, Mathur N, Giles D, Fahlen T, Jones B D. Non-invasive Salmonella typhimurium mutants are avirulent because of an inability to enter and destroy M cells of ileal Peyer’s patches. Mol Microbiol. 1997;24:697–709. doi: 10.1046/j.1365-2958.1997.3741745.x. [DOI] [PubMed] [Google Scholar]
- 35.Rich J J, Kinscherf T G, Kitten T, Willis D K. Genetic evidence that the gacA gene encodes the cognate response regulator for the lemA sensor in Pseudomoas syringae. J Bacteriol. 1994;176:7468–7475. doi: 10.1128/jb.176.24.7468-7475.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 37.Sanderson K E, Stocker B A D. Salmonella typhimurium strains used in genetic analysis. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1987. pp. 1220–1224. [Google Scholar]
- 38.Schiemann D A, Shope S R. Anaerobic growth of Salmonella typhimurium results in increased uptake by Henle 407 epithelial and mouse peritoneal cells in vitro and repression of a major outer membrane protein. Infect Immun. 1991;59:437–440. doi: 10.1128/iai.59.1.437-440.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart G S A B, Lubinsky-Mink S, Jackson C G, Cassel A, Kuhn J. pHG165: a pBR322 copy number derivative of pUC8 for cloning and expression. Plasmid. 1986;15:172–181. doi: 10.1016/0147-619x(86)90035-1. [DOI] [PubMed] [Google Scholar]
- 40.Wang R F, Kushner S R. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene. 1991;100:195–199. [PubMed] [Google Scholar]