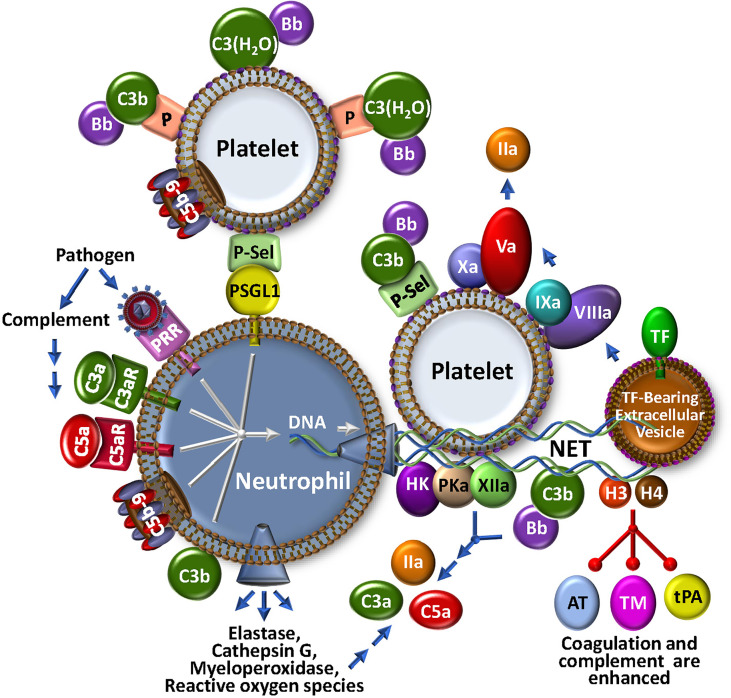
Figure 3.
The NETosis-Coagulation-Complement Web. Neutrophils release NETs after binding of foreign particles to pathogen recognition receptors (PRR), engagement of complement anaphylatoxic or opsonic species, or association of P-selectin on activated platelets with P-selectin glycoprotein 1 (PSGL1). Stimulated neutrophils release numerous bioeffectors including neutrophil elastase, cathepsin G, myeloperoxidase and reactive oxygen species, which may affect production of coagulation and complement bioeffectors directly or indirectly through cell modulation. Release of DNA-based NETs from neutrophils, provides a complex matrix for interactions with stimulated platelets and localizes TF activity, possibly by trapping extracellular vesicles. Both coagulation and complement pathways are further propagated by direct protein factor association with NETs through the contact and alternative pathways, respectively. Multistep crosstalk between these pathways results in further generation of thrombin, C3a and C5a with potential for biological consequence. Histones, H3 and H4, are major protein constituents of NETs and enhance coagulation and complement by inhibiting the regulators, antithrombin (AT) and thrombomodulin (TM), and stabilize the clot by attenuating tPA-mediated fibrinolysis. The activated platelet surface is typically regarded as procoagulant because it provides anionic phospholipid (purple polar head groups), but platelet-bound P-selectin and properdin (P) also stabilize AP C3 convertase assembly via association with C3b and C3(H2O), respectively. The platelet surface may also associate directly with C3(H20) toward complement activation.