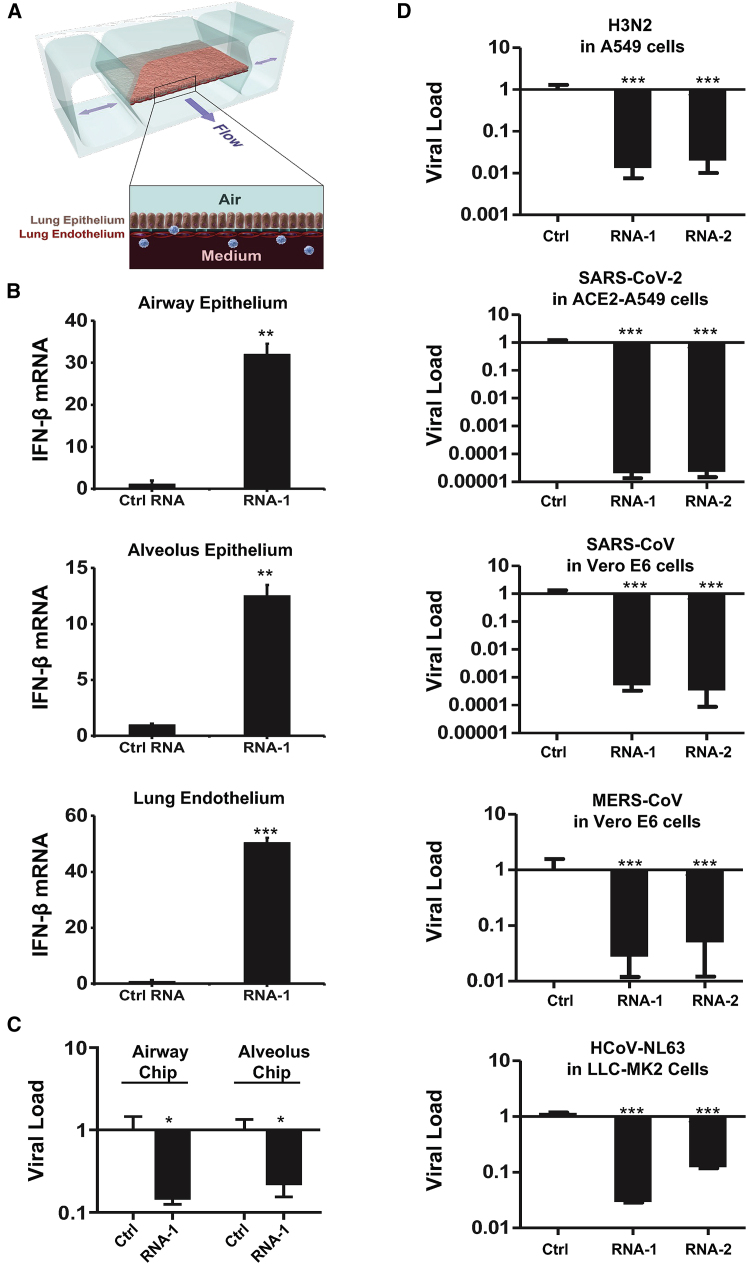
Figure 6.
Immunostimulatory RNAs induce IFN-β production in differentiated human lung epithelial and endothelial cells in organ chips and exhibit broad-spectrum inhibition of infection by H3N2 influenza virus, SARS-CoV-2, SARS-CoV-1, MERS-CoV, and HCoV-NL63
(A) Schematic diagram of a cross section through the human lung on chip, which faithfully recapitulate human lung physiology and pathophysiology. (B) IFN-β mRNA in the epithelial or endothelial cells on human lung airway and alveolus chips at 48 h after transfection with RNA-1 or scrambled RNA control by perfusion through both channels of the chip. Data are presented as fold change relative to the RNA control; N = 3; ∗p < 0.05, ∗∗∗p < 0.001. (C) Effects of treatment with RNA-1 or a scrambled control on viral nucleoprotein (NP) mRNA levels in the human lung airway chips or human lung alveolus chips infected with influenza A/HK/8/68 (H3N2) (MOI = 0.1) at 24 h after RNA-1 treatment. Results are shown as fold change relative to RNA control; N = 3; ∗p < 0.05. (D) Viral load of indicated cells at 48 h after infection after transfection with RNA-1, RNA-2, or a scrambled control for 24 h. For infection, influenza A/HK/8/68 (H3N2) (MOI = 0.1), SARS-CoV-2 (MOI = 0.05), SARS-CoV-1 (MOI = 0.01), MERS-CoV (MOI = 0.01), and HCoV-NL63 (MOI = 0.002), respectively. qPCR in cell lysates was used to quantify viral NP gene for H3N2, and the N gene for SARS-CoV-2 and HCoV-NL63, and plaque-forming assay for SARS-CoV and MERS-CoV. All results are shown as fold change relative to RNA control; N = 3; ∗p < 0.05, ∗∗∗p < 0.001. Data are shown as means ± SD.