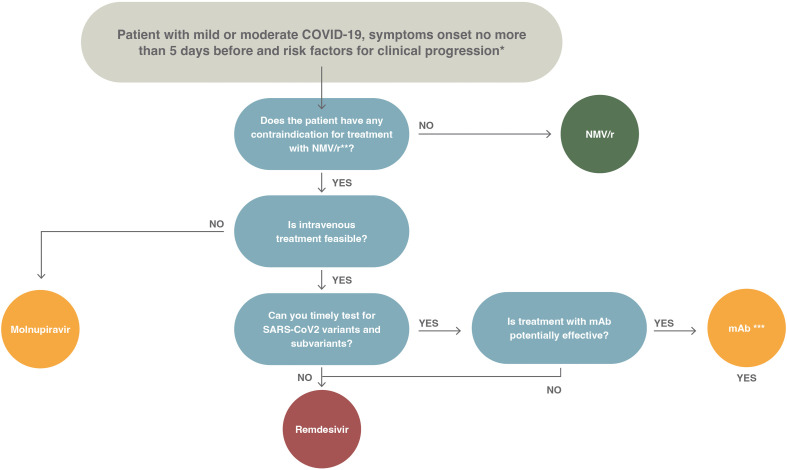
Fig. 2.
Proposed algorithm of treatment for outpatients with mild or moderate coronaviris disease 2019, the onset of symptoms in the previous 5 days, and risk factors for disease progression. COVID-19, coronavirus disease 2019; NMV/r, nirmatrelvir/ritonavir; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
∗The risk factors for disease progression are as follows: ≥60 years of age; body mass index >25 kg/m2; cigarette smoking; immuno-suppressive disease (including human immuno-deficiency virus infection with a CD4 cell count of <200 mm3) or prolonged iatrogenic immunosuppression; chronic lung, cardiovascular, kidney, or sickle cell disease; hypertension; diabetes; cancer; neurodevelopmental disorders or other medically complex conditions or medical-related technological dependence.
∗∗Review potential drug interactions between nirmatrelvir/ritonavir and the patient's current medications. The suggested resources are as follows:
• University of Liverpool. COVID-19 Drug Interactions (https://www.covid19-druginteractions.org/checker)
• National Institutes of Health. Drug-Drug Interactions Between Ritonavir-Boosted Nirmatrelvir (Paxlovid) and Concomitant Medications (https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/ritonavir-boosted-nirmatrelvir--paxlovid-/paxlovid-drug-drug-interactions/#:∼:text=Ritonavir%2C%20a%20strong%20cytochrome%20P450,concentrations%20of%20certain%20concomitant%20medications)
• Infectious Diseases Society of America. Management of Drug Interactions With Nirmatrelvir/Ritonavir (Paxlovid®): Resource for Clinicians (https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/management-of-drug-interactions-with-nirmatrelvirritonavir-paxlovid/)
Careful risk-benefit evaluation in pregnant women and in patients undergoing haemodialysis
∗∗∗Consider the following issues before prescribing monoclonal antibodies: (a) availability of drugs, (b) availability of infusion centres, (c) feasibility to perform timely screening for baseline antibodies, (d) alternatively constant monitoring of the circulation of variants of concern in order to select the appropriate treatment or (e) exclude patients in whom the administration of monoclonal antibodies may be ineffective.