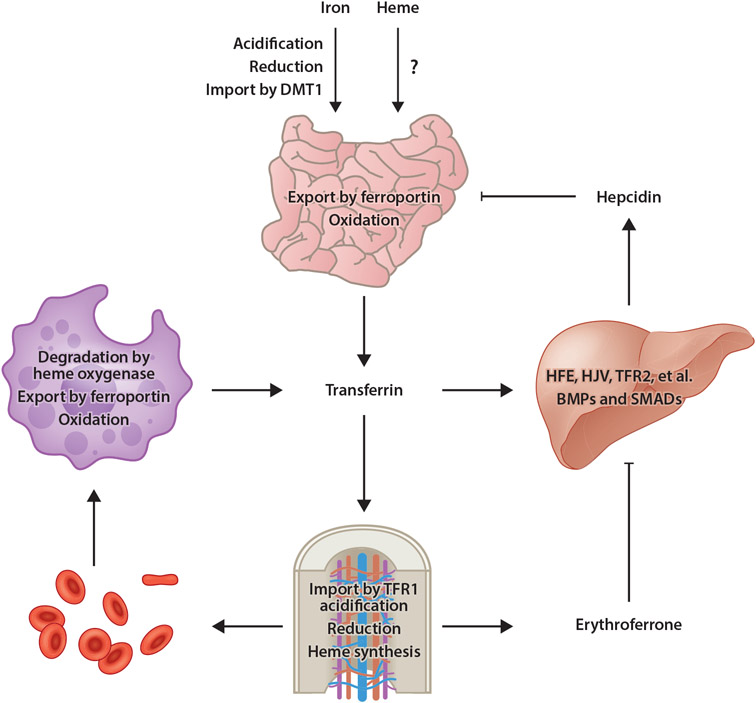
Figure 1.
Model of mammalian iron homeostasis. Both nonheme and heme iron are absorbed in the small intestine. Transporters involved with heme iron absorption have yet to be identified. Nonheme iron is acidified in the stomach, reduced by gastric acid or DCYTB in the small intestine, then imported into enterocytes by DMT1. Iron is then utilized by or stored in enterocytes or exported into circulation by ferroportin. After oxidation by hephaestin, iron binds to transferrin for distribution throughout the body. Most transferrin-bound iron is imported by TFR1-mediated endocytosis into erythroid precursors, a process that requires acidification to liberate iron from transferrin then export into the cytosol followed by reduction by STEAP3. Iron is then utilized for heme synthesis. Senescent or damaged RBCs are degraded by RES macrophages, in which heme oxygenase degrades heme, thereby liberating iron for export by ferroportin into circulation. Under conditions of iron excess, transferrin (and other factors) stimulates BMP expression in sinusoidal endothelial cells. BMPs then stimulate hepcidin expression by hepatocytes in a pathway dependent upon HFE, HJV, TFR2, and other membrane factors, as well as SMAD1/5/8 transcription factors. Hepcidin then posttranslationally inhibits ferroportin activity and expression. Under conditions of anemia, erythroid progenitors produce the hormone erythroferrone, which inhibits BMP-dependent hepcidin expression, thereby ensuring continued dietary iron absorption for erythropoiesis. Abbreviations: BMP, bone morphogenetic protein; DCYTB, duodenal cytochrome b reductase 1; DMT1, divalent metal transporter 1; HFE, homeostatic iron regulator; HJV, hemojuvelin; RBC, red blood cell; RES, reticuloendothelial system; TFR1, transferrin receptor 1; TFR2, transferrin receptor 2. Figure adapted from images created with BioRender.com.