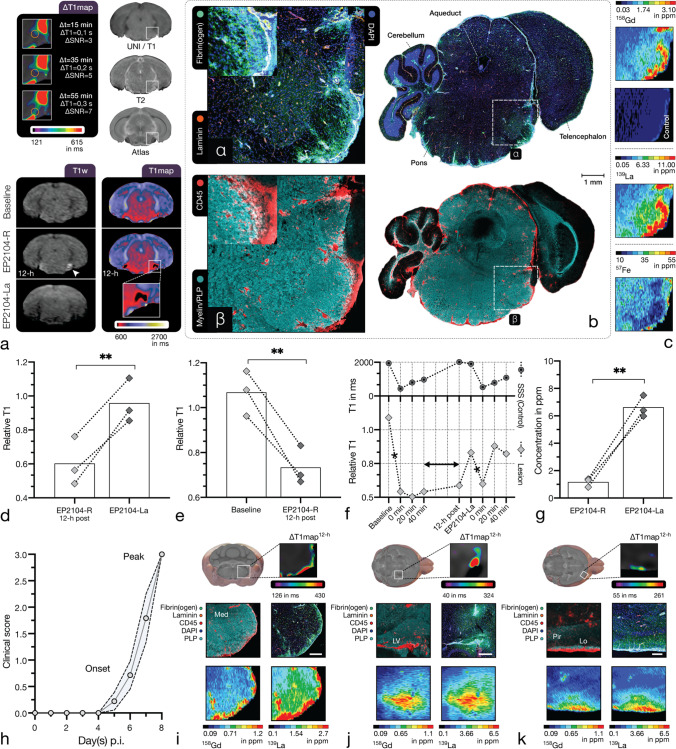
Fig. 3.
Competitive fibrin binding (in vivo) assay. Competitive binding of EP2104-R to the target protein was evaluated in adoptive-transfer EAE mice at disease peak using a lanthanum-labelled analogue (EP2104-La). Panel a shows a representative pontine EAE lesion with prolonged Gd uptake. More than 12-h after the administration of EP2104-R, fibrin-specific contrast enhancement (white arrow) is still apparent, which is displaced with the administration of EP2104-La at tenfold higher dose due to competitive binding. The following panels demonstrate correlated IF-CM (b) and LA-ICP MS (c). Extensive fibrin(ogen) deposits (α) in the brainstem were co-localised with CD45-positive immune cell infiltrates and mild demyelination (β). Within EAE lesions, there was a decrease (p = .008, n = 3, paired one-tailed t-test, E2.3) in relative longitudinal relaxation time between baseline and 12-h post-contrast scans (see e). When an excess of EP2104-La, an MRI-inactive analogue of EP2104-R, was administered, an increase (p = .006, n = 3, paired one-tailed t-test, E2.4) in relative longitudinal relaxation time was apparent (panel d), consistent with displacement of EP-2104-R from its fibrin binding site, as shown in panel f. Mean relative longitudinal relaxation time of an EAE lesion is shown over time. T1 values within the superior sagittal sinus (SSS) show venous contrast dynamics for comparison. Asterisk indicates administration of EP2104-R. Correspondingly, LA-ICP MS of tissue slides showed a statistically significant difference (p = .003, n = 3, paired one-tailed t-test, E2.5) between Gd (EP2104-R) and La (EP2104-La) in EAE lesions (see g). Panel h shows a typical disease course in adoptive-transfer EAE (n = 9; pooled from 2 experiments; dotted line, SEM). The following panels (i–k) show common EAE lesions with corresponding fibrin binding several hours after the administration of EP2104-R. Leptomeningeal inflammation and fibrin binding with extensive myelin damage within the medulla (Med) are shown in panel i. Centripetal distribution of fibrin(ogen) and immune cell clustering indicates that inflammation initiates from the meninges (and small perforating venules). Next panel (j) shows a prevalent pattern with inflammation and CNS migration through the choroid plexus into periventricular regions (LV, lateral ventricle). Last panel (k) illustrates a cortical lesion with subpial and leptomeningeal fibrin deposition with associated immune infiltration and myelin damage affecting the lateral olfactory tract (Lo) and the piriform cortex (Pir). Scale bars, 1 mm (b), 500 µm (i, j), 250 µm (k). p-value < 0.05 was considered statistically significant. **p-value < .01