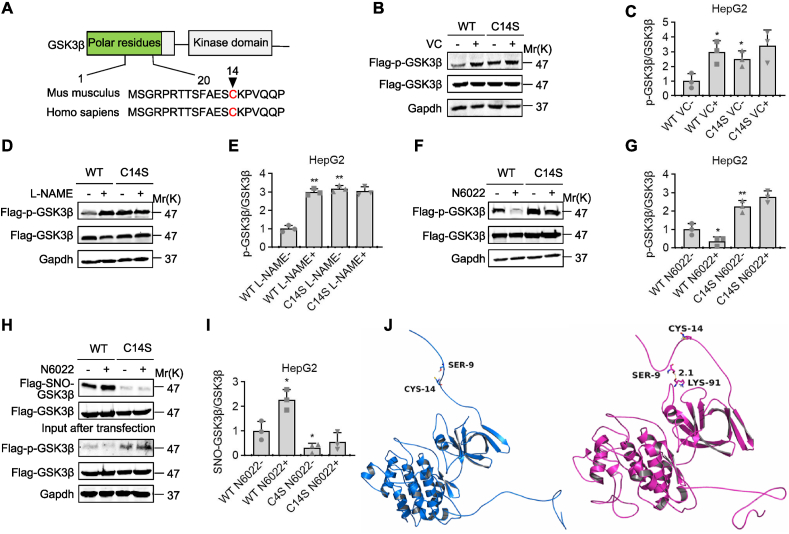
Fig. 6.
GSK3β was denitrosylated and inactivated because of VC. (A) Protein structure and homology sequence analysis of human and mouse GSK3's first 20 amino acids. (B-G) HepG2 cells were transfected with Flag-tagged wild-type GSK3β (WT) or its C14S mutant, then treated for 30 min with 60 μM VC, 6 h with 3.2 mM l-NAME, or 1 h with 5 nM N6022. Protein samples were immunoprecipitated with a Flag antibody before being subjected to western analyses and semi-quantification. (H and I) HepG2 cells were transfected with WT or C14S mutant, then treated for 1 h with 5 nM N6022. Protein samples were immunoprecipitated with a Flag antibody and then subjected to iodoTMT labeling to detect SNO-GSK3β levels, western blotting, and semi-quantification. (J) GSK3β structural modeling following S-nitrosylation at Cys14. The figures were created using Amber14 and 20-ns molecular dynamics simulations based on S-nitrosylation. Left panel: wild-type GSK3β; right panel: Cys14-S-nitrosylated GSK3β obtained from a 20-ns MD simulation. Mean SD, n = 3, one-way ANOVA and Dunnett's multiple comparison tests; *p < 0.05, **p < 0.01 versus lane 1.