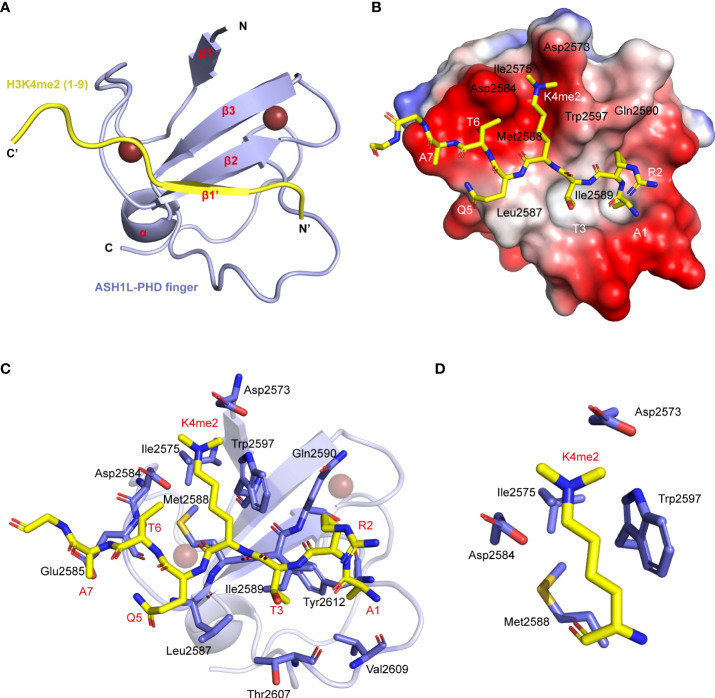
Figure 2.
Structure of ASH1L PHD finger binding with the H3 (1–15) K4me2 peptide. (A) Cartoon representation of the NMR structure of the ASH1L PHD finger (light blue)/H3K4me2 peptide (yellow). (B) Electrostatic potential surface representation of the ASH1L PHD finger binding with H3K4me2 peptide in stick depiction (color-coded by atom type). The amino acid around the K4 residue and in the β2 strand are labeled. (C) The key residues involved in the intermolecular interactions at the protein/peptide interface are labeled. These side chains are color-coded by the atom type and showed in the stick model. (D) The key side chains of binding groove around K4me2 are picked out.