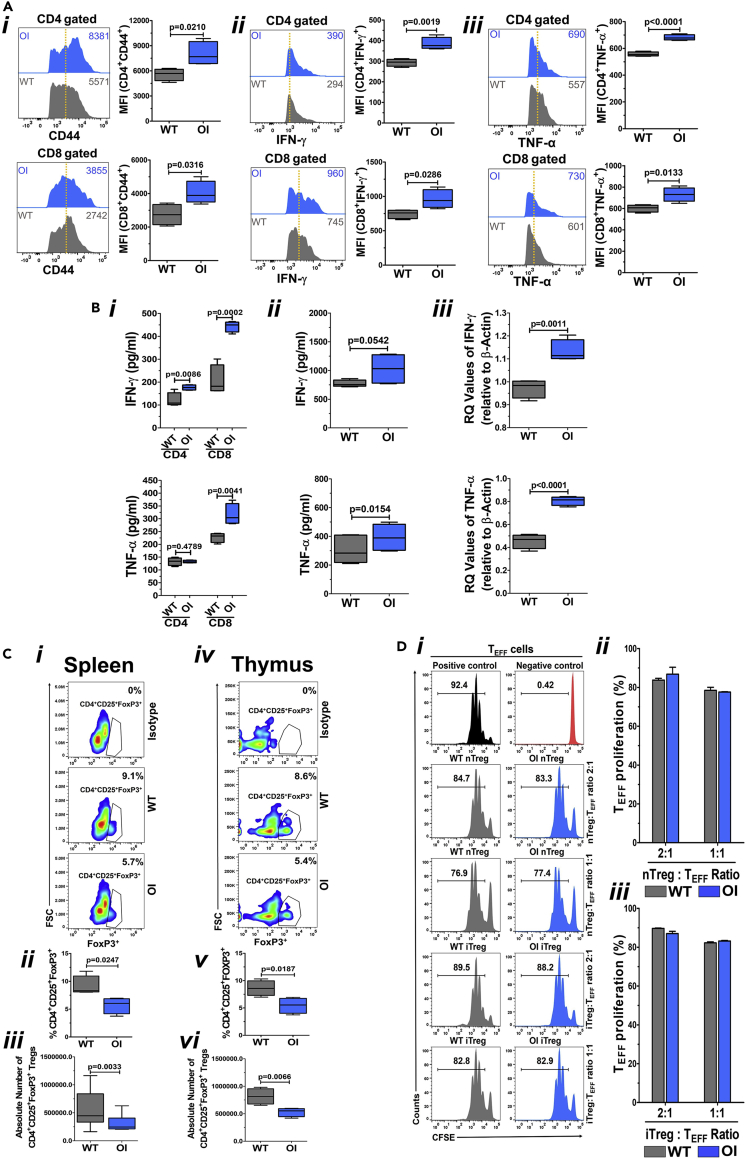
Figure 1.
Evaluation of T cells and Tregs in OI mice
(A) Activated phenotype of T cells and enhanced effector cytokine secretion by T cells in OI mice. Spleen-derived naive T cells from WT and OI mice were stained using fluorochrome-conjugated CD44 antibody, acquired by FACS, and analyzed by FlowJo software (Ai). Data from one of three experiments with a similar result are shown in the left panel. The dotted vertical line indicates the mean position of the peak in WT T cells to compare relative expression between samples visually. Mean MFI values (from all experiments) for CD44 in OI T cells are significantly greater than those seen in WT T cells (right panel). This can be seen in both CD4 (upper panel) and CD8 (lower panel) cells. Splenic T cells from WT and OI mice were activated for three days using anti-CD3 and anti-CD28 antibodies before being re-stimulated with similar stimuli in the presence of Golgi-Plug. Intracellular staining was done with fluorochrome-conjugated antibodies, and data were acquired using FACS. IFN-γ is shown in (Aii), whereas TNF-α is shown in (Aiii). Data from one of three experiments with a similar result are shown in the left panels. The numerical values within each display are MFI, and the dotted vertical line indicates the mean position of the peak in WT T cells. Mean MFI values (from all experiments) show that there is a significantly greater expression of both IFN-γ and TNF-α in OI T cells as compared with WT T cells (right panels) in both CD4 (upper panel) and CD8 (lower panel) cells. Data are presented as Mean ± SD.
(B) Secretion of pro-inflammatory cytokines. Media from splenocyte derived CD4 and CD8 cell cultures was collected and IFN-γ and TNF-α levels were analyzed using ELISA assay (Bi). There is a significant increase in IFN-γ levels in both CD4 and CD8 OI T cell cultures as compared with WT T cell cultures (upper panel). TNF-α levels were significantly increased in only CD8 OI T cells compared with WT T cells (lower panel). IFN-γ and TNF-α levels were also analyzed in serum of WT and OI mice (n = 4) using ELISA assay (Bii). A trend for significant increase is seen in IFN-γ levels (upper panel), whereas a significant increase is seen in TNF-α levels (lower panel) in OI mice serum compared with WT mice serum. mRNA expression of IFN-γ and TNF-α was analyzed in splenocytes using real-time PCR (Biii). A significant increase is seen in both IFN-γ (upper panel) and TNF-α (lower panel) expression in OI mice splenocytes compared with WT mice splenocytes. Data are presented as Mean ± SD.
(C) Reduced number of Tregs in OI mice. Cells from the spleen (Ci, ii, and iii) and thymus (Civ, v, and vi) were stained using fluorochrome-conjugated CD4, CD25, and FoxP3 antibodies to compare the percent of Tregs between WT and OI mice. Flow cytometric plots of the representative data (Ci and iv), and the graph of the cumulative data of percentages of Treg (Cii and v) as well as the absolute number of Tregs (Ciii and vi), demonstrates that there is a significant decrease in the number of Tregs both in the spleen (Ci, ii, and iii) and the thymus (Civ, v, and vi) of OI mice compared with WT mice. Data are presented as Mean ± SD.
(D) Similar Treg functionality in WT and OI mice. nTregs and iTregs from spleens of WT and OI mice were co-cultured with spleen T cell (TEFF; responder cells) from WT mice (labeled with CFSE), in a ratio of 1:1 and 1:2, and stimulated with anti-CD3 and anti-CD28 antibodies for three days. For controls, CFSE labeled WT T cells were cultured in the absence of anti-CD3 and anti-CD28 antibodies. After three days, cells were stained for CD4 and analyzed for CFSE dilution by flow cytometry. nTregs, as well as iTregs from both WT and OI mice, suppressed T cells to the same extent, at both the ratios, as can be seen in flow cytometry plots from a representative experiment (Di) and the graphs of the cumulative data for nTregs (Dii) and iTregs (Diii). Data are presented as Mean ± SD.