Cover Illustration:
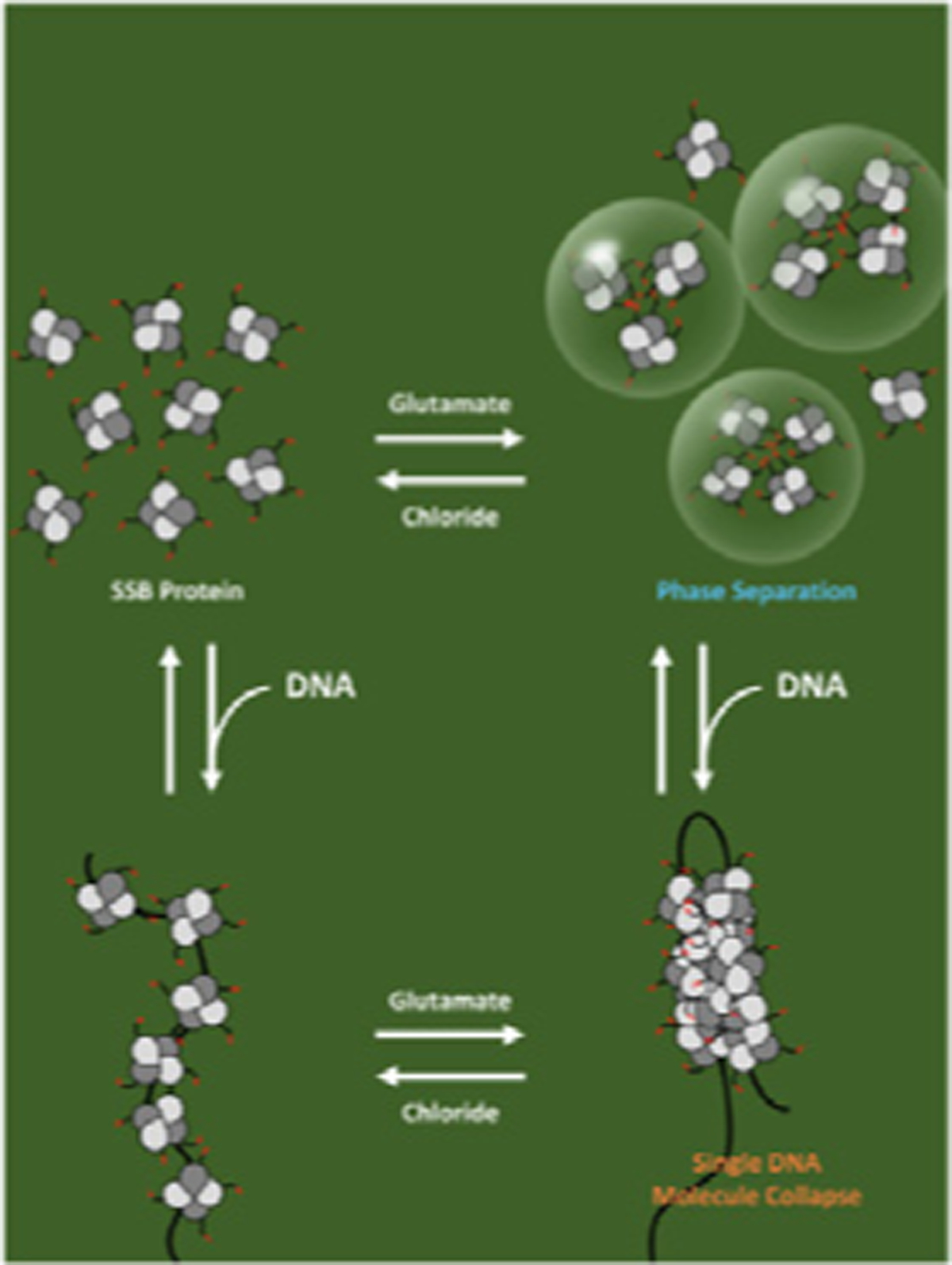
E. coli SSB protein, an essential replication and repair protein, can form a biomolecular condensate via phase separation in the absence of DNA. Phase separation is promoted by potassium glutamate (KGlu), which is the major physiological monovalent salt in bacteria, however, single stranded DNA binding to SSB inhibits this phase separation. However, when SSB is bound to polymeric single stranded DNA, KGlu promotes cooperative binding of SSB on the DNA and compaction of single DNA molecules. The non-physiological salt, KCl, inhibits both phase separation and DNA collapse. The equilibria in the presence of KGlu are proposed to be used to regulate SSB function in genome maintenance. The cover refers to the JMB article in this issue by A.G. Kozlov et al., “How Glutamate Promotes Liquid-liquid Phase Separation and DNA Binding Cooperativity of E. coli SSB Protein,” Volume xxx, Issue xx. The figure was made by Kacey Mersch.
