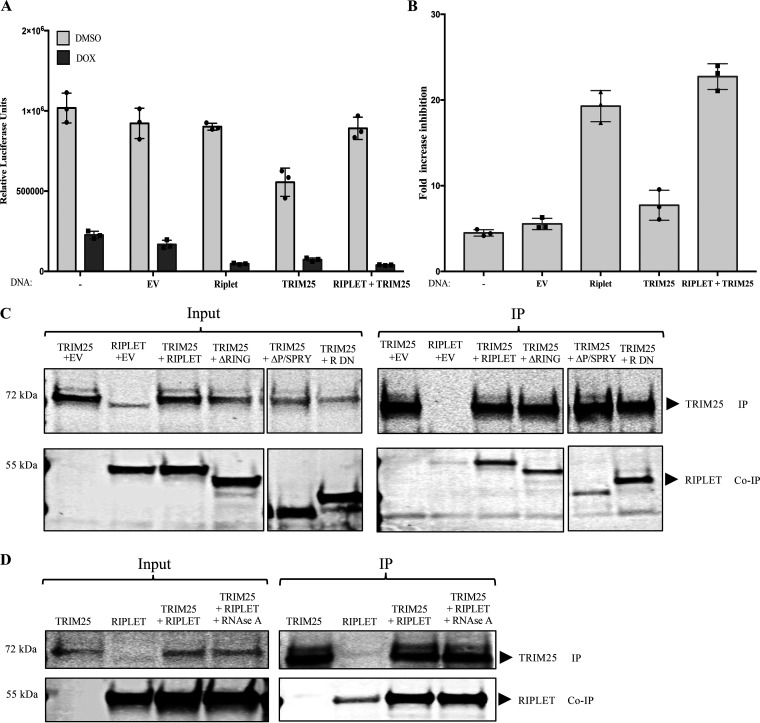
FIG 7.
Riplet and TRIM25 both enhance ZAP activity and coimmunoprecipitate. (A) Effect of Riplet and TRIM25 on ZAP-mediated restriction of HIV-1 reporter expression. 293TrexhZAP cells were left untreated (−) or transfected with empty vector (EV) or DNAs expressing the indicated proteins and then infected with HIV-luc reporter virus. At 6 h postinfection, cells were treated with doxycycline to induce ZAP expression (dark bars) or DMSO as a control (light bars). Firefly luciferase reporter activity was measured at 24 h postinfection and normalized to total protein content measured by a Bradford assay for each sample. Data points presented are the mean RLU/mg ± SD values of three independent experiments done in triplicate. (B) Fold ZAP-mediated inhibition of HIV-1 expression in Riplet- and TRIM25-expressing cells. The fold inhibition was calculated from data in panel A. (C) Coimmunoprecipitation of TRIM25 and Riplet. 293TrexhZAP cells were transiently transfected with DNAs expressing the indicated proteins. Lysates were prepared 48 h later and analyzed directly or subjected to immunoprecipitation. (C, Left) In the input data, total proteins were analyzed by gel electrophoresis, blotted, and probed with α-TRIM25 antibody (top) or anti-Riplet antibodies (bottom). (C, Right) In the IP data, TRIM25 was recovered by immunoprecipitation with α-TRIM25 antibody, and bound proteins were analyzed by electrophoresis and probed for TRIM25 (top) or Riplet (bottom). Molecular weights of major protein estimated from size markers are indicated on left. (D) Coimmunoprecipitation of Riplet and TRIM25 is RNase resistant. 293TrexhZAP cells were transiently transfected by the indicated cDNAs or an empty vector control. Lysates were prepared 48 h later and treated with RNase A (50 μg/mL). Lysates were then analyzed directly or subjected to immunoprecipitation. (D, Left) In the input data, total proteins were analyzed by gel electrophoresis, blotted, and probed with α-TRIM antibody (top) or Riplet antibodies (bottom). Molecular weight of major protein estimated from size markers are indicated on left. (D, Right) In the IP data, TRIM25 was recovered by immunoprecipitation with α-TRIM25 antibody, and bound proteins were analyzed by electrophoresis and probed for TRIM25 (top) or Riplet (bottom). Approximate molecular weights of major proteins estimated from size markers are indicated on left.