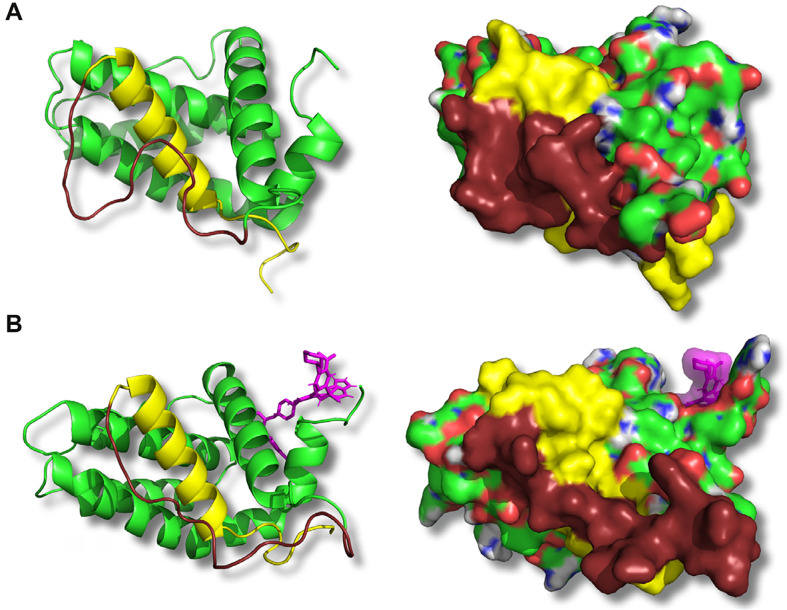
Figure 4.
ABT-199 binding to the BH3 hydrophobic cleft leads to conformational changes on the opposite side of Bcl-2 in the BH4 domain. Representative structures corresponding to the medians of principal structure clusters of the free Bcl-2 (A) or Bcl-2:ABT-199 complex (B) following the ABT-199 binding (in B) and equilibration are shown. Panels show a ribbon representation of the backbone (left) or the CPK-colored surface (right) of the Bcl-2 protein. The BH4 domain is highlighted in yellow, and the adjacent loop is in dark brown. ABT-199 is represented with violet sticks (left) or a semitransparent surface (right). Note a difference in BH4 domain structure (both backbone and exposed surface) as well as adhesion of a part of the loop between BH4 and BH2 domains in the Bcl-2:ABT-199 complex