Figure 3. Δ9-THC-induced cytotoxicity in endothelial cells is associated with inflammation and oxidative stress.
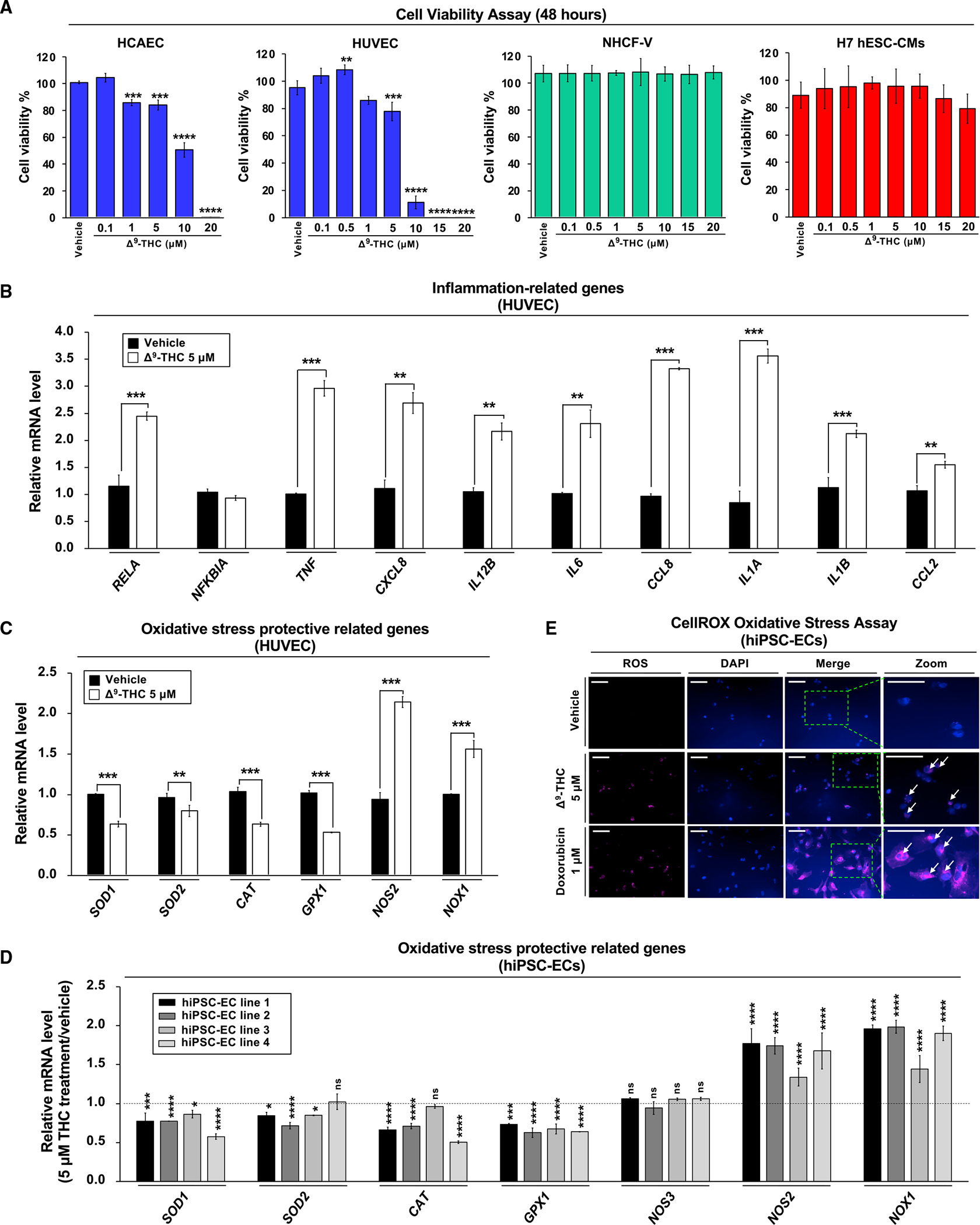
(A) The effects of Δ9-THC on cell viability of human coronary artery endothelial cells (HCAECs), human umbilical vein endothelial cells (HUVECs), normal human cardiac fibroblasts-ventricular (NHCF-V), and human embryonic stem cell-derived cardiomyocytes (hESC-CMs). Cells were treated with increasing concentrations of Δ9-THC for 48 h, and cell viability was measured by the CellTiter-Glo luminescent cell viability assay.
(B and C) (B) The RNA expression of inflammation-related and (C) oxidative stress-related genes in HUVECs. Cells were treated with 5 μM Δ9-THC for 48 h, and gene expression was measured by qPCR and normalized to GAPDH.
(D) Oxidative stress-related gene expression in hiPSC-ECs after Δ9-THC treatment. Cells were treated with 5 μM Δ9-THC for 48 h, and mRNA expression of various genes was measured by qPCR analysis and normalized to GAPDH.
(E) hiPSC-ECs were treated with 5 μM Δ9-THC or 1 μM doxorubicin (positive control) for 48 h and oxidative stress was measured by CellROX oxidative stress assay. Images were obtained by fluorescence microscopy. The white arrowhead indicates cells producing reactive oxygen species (scale bar, 50 μm). Error bars represent mean ± SEM. *p < 0.05 versus vehicle; **p < 0.01 versus vehicle; ***p < 0.001 versus vehicle; ****p < 0.0001 versus vehicle; ns, not significant versus vehicle.
