Figure 6. Genistein blocks Δ9-THC-induced endothelial dysfunction.
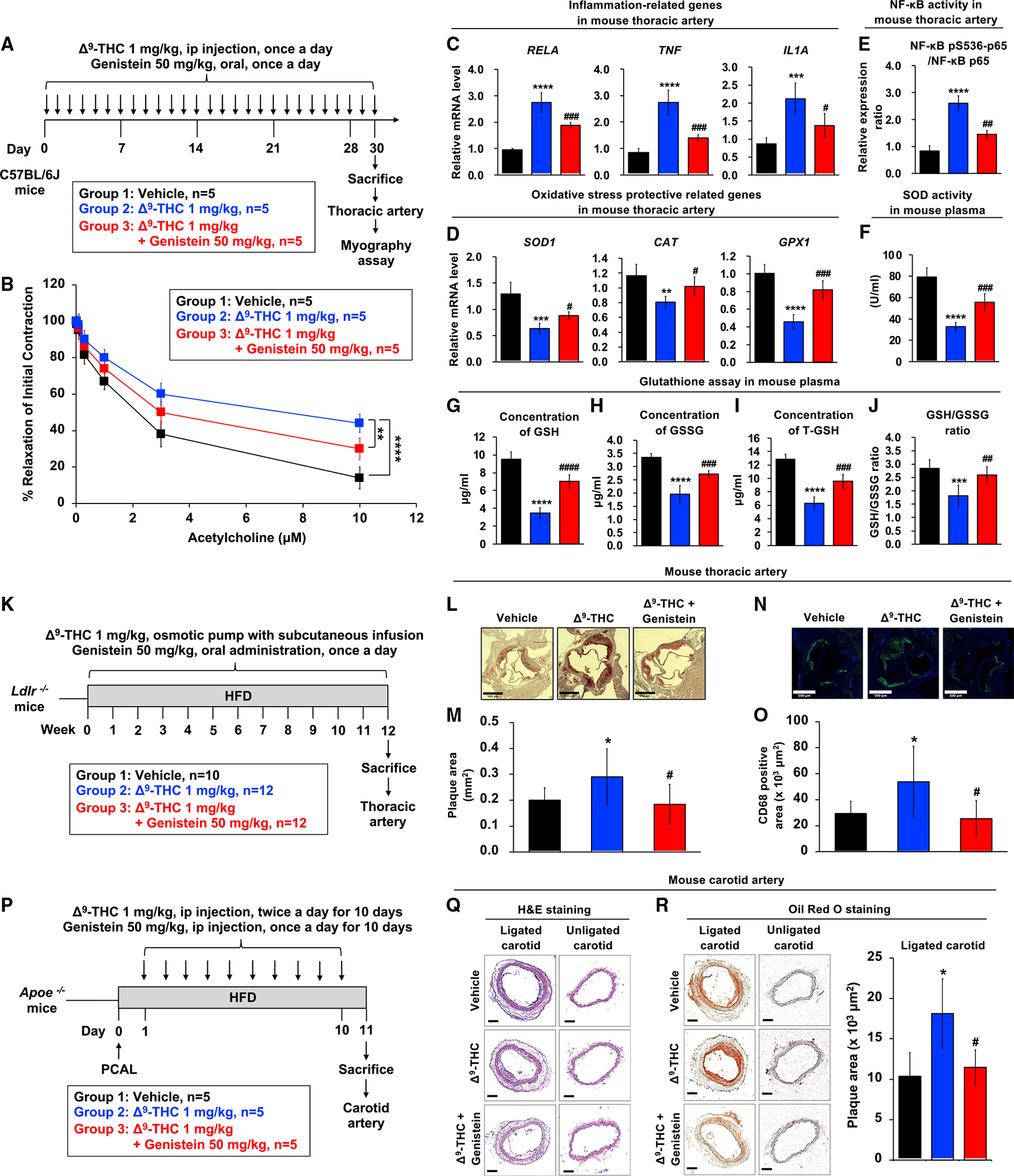
(A) Schematic overview of the wire myograph experimental design in the mouse model.
(B) Isometric tension recordings of isolated mice thoracic aortas were performed using a wire myograph. Vascular concentration-dependent relaxation was induced by acetylcholine (ACh) in pre-constricted mouse thoracic arteries.
(C and D) (C) The mRNA expression of inflammation-related genes and (D) oxidative stress protective-related genes in thoracic artery tissues from mice is shown after normalizing to GAPDH.
(E) Effect of Δ9-THC and genistein on NF-κB phosphorylation in mouse thoracic artery. Total cell lysates were prepared, and the expressions of phosphor-NF-κB were analyzed by ELISA.
(F) Superoxide dismutase (SOD) activity of serum from mouse.
(G) Reduced glutathione (GSH) levels in the serum samples of mice were detected. Plasma isolated from C57BL/6J mice (n = 5) treated with vehicle control, Δ9-THC, genistein, or their combination every day for 30 days was analyzed by the glutathione colorimetric assay kit (BioVision, K261).
(H) Oxidized glutathione (GSSG) levels in the serum samples of mice.
(I) Total glutathione (T-GSH) levels in the serum samples of mice.
(J) The GSH/GSSG ratio in the serum samples of mice.
(K) Schematic overview of the chronic atherosclerosis model. Ldlr−/− mice (9–12 weeks old) were divided into three groups: (1) vehicle control (n = 10), (2) Δ9-THC (n = 12), and (3) Δ9-THC plus genistein (n = 12). Δ9-THC (1 mg/kg/day) or vehicle (90% saline, 5% ethanol, 5% cremophor) was administered subcutaneously using osmotic pumps, and genistein (50 mg/kg/day) or vehicle (corn oil 100 μL/d) was orally administered daily. All experimental animals were fed with a high-fat diet (HFD) for the duration of the treatment protocol. At the end of 12 weeks, the mice were euthanized to determine the extent of atherosclerotic plaque formation.
(L) Oil red O staining of atherosclerotic plaques in cross-sections at the aortic root level with scale bars at 500 μm.
(M) Quantitation of atherosclerotic plaques: (1) vehicle control (n = 10), (2) Δ9-THC (n = 10), and (3) Δ9-THC plus genistein (n = 11).
(N) Immunostaining of CD68 in cross-sections at the aortic root level with scale bars at 500 μm.
(O) Quantitation of CD68-positive area from (1) vehicle control (n = 10), (2) Δ9-THC (n = 9), and (3) Δ9-THC plus genistein (n = 10).
(P) Schematic overview of the experimental design in the Apoe−/− mouse model. Partial carotid artery ligation (PCAL) was performed in Apoe−/− mice (10–16 weeks old). One day after PCAL, Apoe−/− mice were divided into three groups (n = 5/group): (1) vehicle control, (2) Δ9-THC (1 mg/kg intraperitoneally, twice daily for a total of 10 days), and (3) Δ9-THC (1 mg/kg intraperitoneally, twice daily for a total of 10 days) plus genistein (50 mg/kg intraperitoneally, once daily for a total of 10 days). All experimental animals were fed with a high-fat diet (HFD) following PCAL. After 10 days of HFD and treatment exposure, carotid atherosclerosis plaque burden was assayed in all three groups.
(Q) Carotid artery sections were counterstained with hematoxylin and eosin (H&E), and a representative slide was presented with scale bars at 100 μm.
(R) Oil red O staining of atherosclerotic plaques in cross-section of mouse carotid artery (lower panel) with scale bar at 100 μm. The atherosclerotic plaques were quantified. Error bars represent mean ± SEM. *p < 0.05 versus vehicle; **p < 0.01 versus vehicle; ***p < 0.001 versus vehicle, ****p < 0.0001 versus vehicle; ns, not significant versus vehicle; #p < 0.05 versus Δ9-THC; ##p < 0.01 versus Δ9-THC; ###p < 0.001 versus Δ9-THC; ####p < 0.0001 versus Δ9-THC.
